1055
UTE MRI can detect myelin loss in mice using an open field low intensity blast injury model of mild traumatic brain injury (mTBI)1UC San Diego, San Diego, CA, United States, 2Missouri University of Science and Technology, Rolla, MO, United States, 3VA Health System, San Diego, CA, United States
Synopsis
Mild traumatic brain injury (mTBI) may cause significant myelin damage, leading to significant degradation of elaborate cognitive functions. However, conventional neuroimaging techniques are unable to accurately assess myelin, and fail to show abnormalities in the majority of mTBI cases. UTE MRI sequences with echo times (TEs) <0.1 ms allow direct imaging and quantitative assessment of myelin density. Here we aim to investigate whether the 3D IR-UTE sequence can detect myelin loss in mice using an open field low intensity blast injury model of mTBI. This technique provides a new approach for potentially more accurate diagnosis and treatment monitoring of mTBI.
Introduction
Mild traumatic brain injury (mTBI) is a major cause of long-term disability, with an annual 1.7 million Americans sustaining non-fatal TBI. Shear strains due to linear and rotational acceleration of the brain can severely damage axons and their myelin sheaths (1-3). Myelin is particularly vulnerable to secondary damage as a result of chemical cascades and neuroinflammation (1-3). Myelin impairment can disrupt axonal transport, integrity, and plasticity, leading to a massive reduction in signal transduction (4-6). Given its indispensable role in the development and maintenance of elaborate cognitive functions, loss of myelin could play a key role in the pathogenesis of mTBI. However, conventional neuroimaging techniques are unable to accurately assess myelin, and fail to show abnormalities in the majority of mTBI cases (7-9). Ultrashort echo time (UTE) MRI sequences with echo times (TEs) <0.1 ms allow direct detection of signals from myelin (10-14). In this study, we aim to investigate whether 3D UTE MRI can detect myelin loss in mice using an open field low intensity blast injury model of mTBI.Methods and Materials
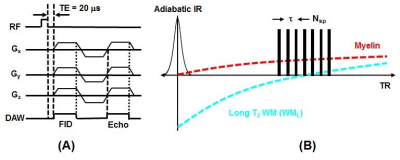
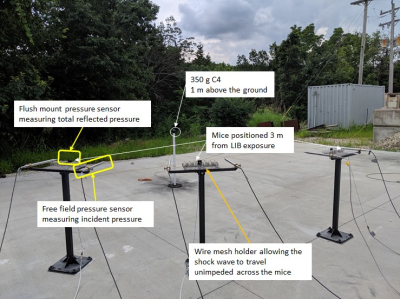
A 3D adiabatic inversion recovery prepared UTE (3D IR-UTE) sequence (Figure 1) was implemented on a 7T Bruker horizontal MRI system with 1,000 mT/m strength and 11,250 T/m/s slew rate. An adiabatic IR pulse is used to invert the longitudinal magnetizations of long T2 white matter (WML) (11-13). During this pulse, the longitudinal magnetization of myelin is not inverted, but is largely saturated due to its ultrashort T2. UTE data acquisition starts around the TI necessary for the inverted longitudinal magnetization of WML to reach its null point, leaving signals from myelin and some residual long T2 tissues to be detected by the FID. The second echo acquires signals from any residual long T2 tissues with zero signal from myelin. Subtraction of the second echo image from the first one provides selective imaging of myelin (11-13). The 3D IR-UTE sequence employed the following parameters: TR = 1000 ms, inversion time (TI) = 350 ms, TE = 0.02/2.0 ms, field of view (FOV) = 2.2×2.2×2.2 cm3, matrix = 128×128×128 cm3, flip angle (FA) = 20º. A total number of 21 spokes was acquired per IR preparation, leading to a total scan time of 100 min.A total of 6 male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) at ~8 weeks of age were studied according to the institutional guidelines. Mice were divided into mTBI (n=3) and sham (n=3) groups. The mTBI group were subject to a recently established highly reproducible open-field LIB injury murine model (15), where anesthetized mice were placed in the prone position three meters away from a detonation of 350g high-energy explosive C4 (both 1 m aboveground) (Figure 2). Sham mice underwent identical procedures, but without blast exposure. Four days later the animals were scanned with the 3D IR-UTE sequence followed by histological myelin assessment with Luxol Fast blue (LFB) staining. UTE measured myelin density was correlated with LFB myelin staining.
Results and Discussion
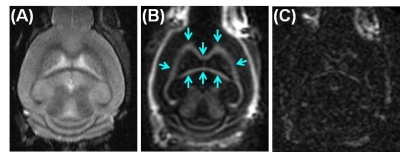
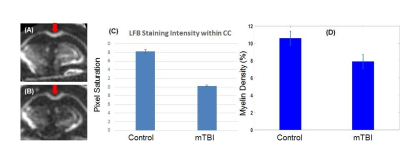
Figure 3 shows coronal T2-FSE and IR-UTE imaging of a normal mouse. Cortical bone in the skull and myelin in white matter are depicted with high signal and contrast on the IR-UTE images, but are invisible using FSE imaging.Figure 4 shows 3D IR-UTE imaging of a control C57BL/6 mouse and an mTBI mouse four days post an open-field LIB injury. About 25% myelin loss was observed in the CC of mTBI mice, as confirmed by LFB results.
We have demonstrated that that 3D IR-UTE sequences can detect myelin loss in mice induced by the open-field LIB injury model. More recently, myelin sheath defects were identified in the corpus callosum (CC) of mice subject to low-intensity blast (LIB) exposure, a highly reproducible open-field LIB injury murine model of mTBI (15). Myelin defects appeared as extensive split layers, dense degeneration, myelin ballooning, myelin disruption or myelin detachment at 7 days post-blast injury (DPI), and returned to normal appearance 30 days DPI, consistent with evidence from other related research (15). Our results were largely consistent with the reported results, and further confirmed that the 3D IR-UTE sequence could be used to monitor myelin loss in mice subject to the open-field LIB injury, which might be more robust than the traditional controlled cortical impact model of mTBI. A systematic study of two larger groups of mice (normal vs. mTBI induced by the LIB injury) will be performed, and will likely confirm the technical capability in evaluation of demyelination and remyelination in mice subjected to mTBI, and their association with behavioral testing.
Conclusion
The 3D IR-UTE sequence allows quantitative imaging of myelin in mouse brain, and can reliably measure myelin loss in white matter of the brain induced by the open-field low intensity blast. This technique provides a new approach for potentially more accurate diagnosis and treatment monitoring of mTBI.Acknowledgements
The authors acknowledge grant support from the NIH (R01NS092650, R01AR075825 and R21AR075851), Veterans Affairs (Merit Awards I01CX001388 and I01RX002604), and GE Healthcare.References
1. Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: clues to axon and myelin repair capacity. Experimental Neurology 2016; 275:328-333.
2. Jurick SM, Bangen KJ, Evangelista ND, Sanderson-Cimino M, Delano-Wood L, Jak AJ. Advanced neuroimaging to quantify myelin in vivo: application to mild TBI. Brain Inj. 2016; 30:1452-1457.
3. Weber AM, et al. Imaging the role of myelin in concussion. Neuroimaging Clin N Am 2018; 28:83-90.
4. Sinha K, Karimi-Abdolrezaee S, Velumian AA, Fehlings MG. Functional changes in genetically demyelinated spinal cord axons of shiverer mice role of juxtaparanodal Kv1 family K+ channels. Journal of Neurophysiology 2006; 95:1683-1695.
5. Lee Y, Morrison BM, Li Y., et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012; 487:443-448.
6. Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001; 294:1731-1735.
7. Bigler ED, Orrison WW. Neuroimaging in sports-related brain injury. Eds. Lovell MR, Echemendia RJ, Barth JT, Collins MW. Pp. 71-94. Lisse, Netherlands: Swets and Zeitlinger 2004.
8. Kirkwood WM, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics 2006; 117:1359-1371.
9. Johnston KM, Ptito A, Chankowsky J, et al. New frontiers in diagnostic imaging in concussive head injury. Clin J Sport Med 2001; 11:166-175.
10. Horch RA, Gore JC, Does MD. Origins of the ultrashort T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med 2011; 66:24-31.
11. Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci USA 2012; 109:9605-9610.
12. Du J, Ma G, Li S, Carl M, Szeverenyi N, VandenBerg S, Corey-Bloom J, Bydder GM. Ultrashort TE echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. NeuroImage 2013; 87C:32-41. PMID: 24188809.
13. Ma Y, Jang H, Wei Z, Cai Z, Xue Y, Chang EY, Corey-Bloom J, Du J. Robust human brain myelin imaging using a short TR adiabatic inversion recovery prepared ultrashort echo time (STAIR-UTE) Sequence. Radiology 2020; 297:392-404.
14. Weiger M, Froidevaux R, Baadsvik EL, Brunner DO, Rosler MB, Pruessmann KP. Advances in MRI of the myelin bilayer. Neuroimage 2020; 217:116888.
15. Song H, Konan LM, Cui J, Johnson CE, et al. Ultrastructural brain abnormalities and associated behavioral changes in mice after low-intensity blast exposure. Behavioural Brain Research 2018; 347:148-157.
Figures