1049
Neurochemical characteristics of pathological tissues in epilepsy: a 1H MRS study at 7T1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Animal Imaging and Technology, EPFL, Lausanne, Switzerland, 3Division of Neuroradiology, Diagnostic Department of Geneva University Hospitals and University of Geneva, Geneva, Switzerland, 4Division of Neurology, Neurosciences Department of Geneva University Hospitals and University of Geneva, Geneva, Switzerland, 5Center for Biomedical Imaging of Geneva and University of Geneva, Geneva, Switzerland
Synopsis
This study is aim to evaluate the neurochemical characteristics of pathological tissues by 1H MRS in patient with epilepsy at 7T. In comparison to the contralateral side, lesions in focal cortical dysplasia demonstrated significantly reduced macromolecule and N-acetyl aspartate, significantly increased total choline and glycine + myo-inositol, and a distinct reduction trend of glutamate. We conclude that performing MRS at high magnetic field offered the potential to reveal novel metabolic alterations in epilepsy lesions that may help to further understand the underlying pathophysiology of the disease.
Introduction
1H magnetic resonance spectroscopy (MRS) studies in epilepsy have been largely performed at 3T. Although high magnetic field strengths have shown great potential for improving the detection of metabolites, research studies in epilepsy at 7T are sparse1. The aim of this study is to evaluate the neurochemical characteristics of pathological tissues by 1H MRS in patients with epilepsy at 7T.Methods
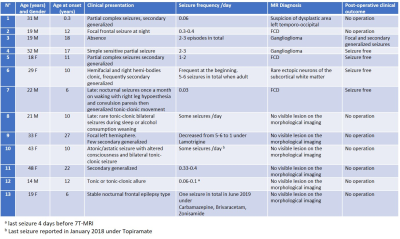
Thirteen patients (demographic data in Table 1) known for drug-resistant epilepsy provided written informed consent and participated the MR study. All MR experiments were conducted on a 7T/68cm MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a 1H quadrature surface coil or a single-channel quadrature transmit and 32-channel receive coil (Nova Medical Inc., MA, USA) depending on the location of the epilepsy lesion. The dielectric pad filled with a solution of deuterated water and barium titanate was used to enhance local transmit field.T1 weighted morphological images were obtained by MP2RAGE sequence for planning the voxel positioning when performing MRS. For each patient, MR spectra were acquired from two voxels (lesion side and contralateral side) using the sSPECIAL sequence2 with the identical experimental parameters. Depending on the coil used and location of the voxel, the following parameters were used: TE=12/16ms, TR=6.2-8.0s. For patients who do not demonstrate an apparent MRI detectable lesion, the ‘lesion side’ voxel was positioned based on metabolic, electrical or clinical information.
MR spectra were analyzed by LCModel3 and metabolite levels (CRLB<50%) were reported as to total creatine ratio. The change between the lesion and its contralateral side was reported in percentage and calculated by their difference over the value of contralateral side. Two tailed paired t-test was used to compare neurochemical changes between the lesion and contralateral side.
Results
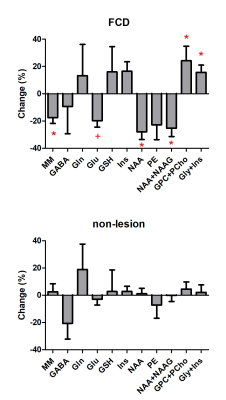
Thirteen patients were diagnosed based on the MRI morphological sequences and histopathology: focal cortical dysplasia (FCD, n=4, one with suspicion), ganglioglioma (n=2), no MR visible lesion (n=6) and rare ectopic neurons of the subcortical white matter (n=1). Due to the small sample size in certain groups, statistical analysis was performed in FCD and non-lesion patient groups.In comparison to the contralateral side, FCD lesion demonstrated significant reductions in macromolecule (MM, -18%) and N-acetyl aspartate (NAA, -28%), significant increases in total choline (GPC+PCho, +24%) and glycine + myo-inositol (+16%), and a distinct reduction trend of glutamate (Glu, -20%, p=0.057). No significant difference was found in non-lesion patients.
Discussion and conclusion
The reduced NAA, increased total choline, and increased myo-inositol + glycine were consistent with previous findings at 3T4. With the increase of spectral resolution and SNR available at 7T, metabolites measurement can be largely improved especially for J-coupled metabolites like glutamate and glutamine. This allows us to detect for the first time, a reduction trend of glutamate in the FCD lesion, which should be further validated with a large sample size.We conclude that performing MRS at high magnetic field offered the potential to reveal novel metabolic alterations in epilepsy lesions that may help to further understand the underlying pathophysiology of the disease.
Acknowledgements
We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole polytechnique fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG). Funding was provided by Startup of the Radiology department.References
1. Voets NL, Hodgetts CJ, Sen A, Adcock JE, Emir U. Hippocampal MRS and subfield volumetry at 7T detects dysfunction not specific to seizure focus. Sci Rep. 2017 Nov 23;7(1):16138.
2. Xin L, Schaller B, Mlynarik V, Lu H, Gruetter R. Proton T1 relaxation times of metabolites in human occipital white and gray matter at 7 T. Magn Reson Med. 2013 Apr;69(4):931-6.
3. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672-9.
4. Tschampa HJ, Urbach H, Träber F, Sprinkart AM, Greschus S, Malter MP, Surges R, Gieseke J, Block W. Proton magnetic resonance spectroscopy in focal cortical dysplasia at 3T. Seizure. 2015 Nov;32:23-9.
Figures