1046
T2 relaxometry and 18F-FDG-PET alterations in hippocampus and hippocampal subfields in left and right MR-negative temporal lobe epilepsy1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Department of Nuclear Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 3Department of Neurosurgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Synopsis
Both PET and T2 relaxometry could provide complementary information of the epileptogenic zone, which could add value to presurgical planning of epilepsy patients. This study investigated evaluted the performance of hippocampual asymmetry measures from volumetry, T2 relaxometry and 18F-FDG PET in lateralization for MR negative left from right temporal TLE. We also investigated how hippocampal subfield alterations of T2 relaxometry and 18F-FDG-PET. Our experimental results showed the combination of T2 relaxometry and PET could complement each other in lateralization for MR-negative LTLE.
Introduction
Temporal lobe epilepsy (TLE) is the most frequent drug-resistant epilepsy [1]. Accurate lateralization of epileptogenic zone is a prerequisite for pre-surgical planning [2-4]. Hippocampal sclerosis (HS) is the common cause or results of TLE, which characterized by neuronal cell loss and gliosis within the hippocampus [5]. Typical radiological characteristics of HS include volume atrophy and increased T2 signal. However, up to 16% to 30% of TLE patients do not have MRI identifiable lesions, even if many exhibit histological features of HS in resected tissue, so-called “MR-negative epilepsy” [6]. \MR-negative TLE patients present a challenge in identifying the epileptogenic zone, which complicates the presurgical workup. A hybrid PET/MR scanner with simultaneous acquisition permits simultaneous imaging of physiological and pathophysiological processes and provides both anatomical and metabolic information on the same subject [7]. In this study, we aim to investigate lateralization ability using hybrid PET/MR imaging of the hippocampus in MR-negative TLE patients with quantitative T2 relaxometry and 18F-FDG-PET.Method
Data acquisition:In this IRB approved study, fifteen MR-negative LTLE patients and ten MR-negative RTLE were recruited, with demographics listed in Table 1. The subjects’ radiological reading and clinical diagnosis were performed by experienced radiologists and neurosurgeons. In addition, fifteen healthy volunteers were recruited for T2 scans and twenty-one for PET scans. The PET and MR scans were performed on a PET/MR scanner (Biograph mMR; Siemens Healthcare, Erlangen, Germany) at Ruijin Hospital, Shanghai, China. The PET images were obtained at 15 minutes post a bolus injection of 18F-FDG (mean dose of 3.7 MBq/kg, matrix size = 344×344, voxel size = 2.0×2.0 ×2.0 mm3, 127 slices). The MR experimental protocols included multi-contrast spin-echo T2-weighted mapping (0.4×0.4×5.0mm3, TE 10.5/21.0/31.5/42.0/52.5/63.0ms, TR 2000ms, matrix size = 256×256, 21 slices) and T1-weighted anatomical images using MPRAGE (1.0×1.0×1.0 mm3, TR/TE = 1900/2.44 ms, matrix size = 256×256, 192 slices).
Data processing and data analysis:
Hippocampus and hippocampal subfields (CA1, CA3, CA4 and DG (GC-ML-DG)) were automatically segmented from the T1-weighted image with the FreeSurfer image analysis v7.0 package (https://surfer.nmr.mgh.harvard.edu). Bilateral hippocampal volumes were quantitatively assessed, with the bilateral hippocampi and subfields extracted as ROIs for further analysis. Hippocampal volumes were normalized with respect to the total intracranial volume [8]. Voxelwise T2 maps were computed by monoexponential fitting of the multi-echo signals. Voxels with T2 values >170 msec within the masks were excluded to minimize cerebrospinal fluid (CSF) contaminations [5]. The FDG uptakes (SUVRs) were obtained using intensity normalization by global mean scaling of 18F-FDG PET images, to correct individual variations in global brain metabolism. We co-registered T2 maps derived from multi-echo PD/T2 image and SUVRs to structural MRI. The segmented hippocampi were further eroded to avoid partial volume effect, then served as ROIs to obtain the corresponding T2 and SUVR values. Z-scores of corrected hippocampal volume, T2 value and SUVR were calculated for each subject in comparison with the control group. The Wilcoxon signed-rank tests were applied to compare Z scored volume, T2, and SUVR on the ipsilateral and contralateral whole hippocampus and hippocampal subfields in LTLE and RTLE groups. Asymmetry indices were defined as AI = left – right (using z-scored values). Logistic regression with univariate and multivariate models were used to evaluate lateralization ability of hippocampal volumes, T2 map and SUVR. Area under the curve (AUC) and accuracy were used for the quantitative assessment of the logistic regression models.
Results and Discussion
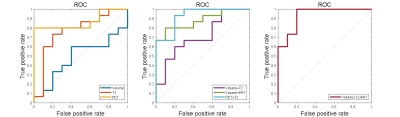
Across all patients, the ipsilateral hippocampi of the SOZ is characterized by increased T2 signal (P<0.01) and decreased FDG uptake (P<0.001) compared to contralateral hippocampi, consistent with the reactive gliosis [9] and mitochondrial dysfunction or neuronal loss [10] as previously reported. Hippocampal volume did not show significance. However, the statistical significance alters if we divide the group by their clinically confirmed laterality: FDG-PET (P < 0.01 for both RTLE and LTLE), T2 relaxometry (P < 0.05 only for RTLE) (Figure 1). For hippocampal subfields, increased T2 were found in the ipsilateral CA3, CA4 and DG of MR-negative RTLE, but no significant T2 change were seen in any ipsilateral subfields of LTLE. Decreased FDG uptakes were found in CA1 and CA4 of MR-negative RTLE, and CA3 and DG of MR-negative LTLE (Figure 1). These differences might be associated with previously reported pathological asymmetry between left and right TLE [11]. Among univariate models, the best performance was FDG-PET SUVR (AUC=0.87), followed by T2 (AUC=0.77), and hippocampal volume (AUC=0.49). Compared to univariate models, the multivariable models (T2+PET and Volume+T2+PET) obtain the highest area under the curve (AUC=0.93) (Figure 2). The combined PET, T2 and hippocampal volume show the best performance in specificity for lateralizing MR-negative TLE. Especially for MR-negative LTLE, the combination of PET and T2 improved the accuracy of lateralization (Table 2).Conclusions
We demonstrated that T2 relaxometry and PET-SUVR alters differently in hippocampus and hippocampal subfields of left- and right- MR negative TLEs. This may contribute to their ability to complement each other in lateralization for MR-negative TLE.Acknowledgements
This study is supported by the Ministry of Science and Technology, Grant No. 2017YFC0109002.References
1. Malmgren K, Thom M. Hippocampal sclerosis--origins and imaging. Epilepsia. 2012;53(4):19-33. 2. Duncan JS, Winston GP, Koepp M J, et al. Brain imaging in the assessment for epilepsy surgery. Lancet Neurol. 2016;15(4):420-433.
3. Spencer DD, Gerrard JL, Zaveri HP. The roles of surgery and technology in understanding focal epilepsy and its comorbidities. Lancet Neurol. 2018;17(4):373-382
4. Zijlmans M, Zweiphenning W, van Klink N. Changing concepts in presurgical assessment for epilepsy surgery. Nat Rev Neurol. 2019;15(10):594-606.
5. Winston GP, Vos SB, Burdett JL, et al. Automated T2 relaxometry of the hippocampus for temporal lobe epilepsy. Epilepsia. 2017;58(9):1645-1652.
6. Muhlhofer W, Tan YL, Mueller SG, et al. MRI-negative temporal lobe epilepsy-What do we know? Epilepsia. 2017;58(5):727-742.
7. Ding YS, Chen BB, Glielmi C, et al. A pilot study in epilepsy patients using simultaneous PET/MR. Am J Nucl Med Mol Imaging. 2014;4(5):459-470.
8. Winston GP, Cardoso MJ, Williams E J, et al. Automated hippocampal segmentation in patients with epilepsy: available free online. Epilepsia. 2013;54(12):2166-73.
9. Bouilleret V, Nehlig A, Marescaux C, et al. Magnetic resonance imaging follow-up of progressive hippocampal changes in a mouse model of mesial temporal lobe epilepsy. Epilepsia. 2000;41(6):642-50. 10. Tenney JR, Rozhkov L, Horn P, et al. Cerebral glucose hypometabolism is associated with mitochondrial dysfunction in patients with intractable epilepsy and cortical dysplasia. Epilepsia. 2014 Sep;55(9):1415-22.
11. Zaidel DW, Esiri MM, Oxbury JM. Regional differentiation of cell densities in the left and right hippocampi of epileptic patients. J Neurol. 1993;240(5):322-5.
Figures