1044
Cerebral Morphometric Alterations in Patients with Temporal Lobe Epilepsy related to Antero-inferior Temporal Meningoencephalocele
Laleh Eskandarian1,2, Safak Parlak3, Gokce Ayhan4, Irsel Tezer4, Serap Saygi4, and Kader Karli Oguz2,3
1Neuroscience Department, Bilkent University, Ankara, Turkey, 2National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey, 3Faculty of Medicine, Department of Radiology, Hacettepe University, Ankara, Turkey, 4Faculty of Medicine, Department of Neurology, Hacettepe University, Ankara, Turkey
1Neuroscience Department, Bilkent University, Ankara, Turkey, 2National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey, 3Faculty of Medicine, Department of Radiology, Hacettepe University, Ankara, Turkey, 4Faculty of Medicine, Department of Neurology, Hacettepe University, Ankara, Turkey
Synopsis
This study investigates morphological alterations in patients with left temporal lobe epilepsy (TLE) due to ipsilateral antero-inferior temporal lobe meningoencephalocele (TLM). Compared with healthy control (HC)s, cortical thickness was increased extensively in both cerebral hemispheres with reduction in a few regions, most remarkably in the left temporal lobe. Both amygdala were significantly bigger in the patients compared with HCs. No difference was found in hippocampi and thalami between patients and HCs or between hemispheres within the patient group. No relation was found between these significant alterations and duration of disease.
Purpose
Although rare, antero-inferior temporal lobe meningoencephalocele (TLM) is increasingly reported as a treatable cause of drug-resistant temporal lobe epilepsy (TLE). Although there is a known relationship between increased intracranial hypertension (IIH) and TLM, the occurrence of these lesions near the skull base sutures and in nonobese patients without IIH suggests its congenital origin. In cases with epilepsy and long-standing cases, a widespread cortical and subcortical abnormality can be anticipated. Therefore, we aimed to search for cerebral cortex alterations as well as hippocampus, amygdala, and thalamus volumes in patients with TLE with TLM which is unrelated to IIH.Methods
IRB was obtained for this retrospective study.Epilepsy-dedicated MRIs of all patients diagnosed with TLE were retrospectively evaluated by two neuroradiologists with an experience on epilepsy imaging to include those with TLM in absence of IIH. Twelve patients with left TLM (F/M, 6/6; Mean age ± SD, 28.00 ± 13.21) and 39 matched healthy control (HC)s (F/M, 12/27; Mean age ± SD, 32.03 ± 9.02) were included in the study. All participants had a 3D T1-weighted FFE (TR/TE = 7.9 / 3.5 ms, FA:450, 1mm slice thickness with %25 overlap) imaging obtained on a 1.5T scanner (Achieva, Philips, Netherlands).
Data Processing and Analysis:
Structural analyses were performed using the Freesurfer image analysis package version 6.0.0. Preprocessing steps included removal of non-brain tissue, subcortical segmentation1, and identification of white matter/gray matter boundary based on the cortical reconstruction and volumetric parcellation. In this study, Freesurfer dev version was used to segment the hippocampus2, amygdala3, and thalamus-nuclei4. The segmentations mentioned above were conducted by using the T1-weighted NIFTI files. Parcellation, annotation, and anatomical statistical scripts of Freesurfer were used to find the subcortical volumes and hemispheric cortical thicknesses. In the next step, we prepared data for group analysis. In the first step, using recon-all, the images were preprocessed, then the FSGD and contrast file was created. In order to group analysis, a single dataset was prepared according to our individual structural maps. The data were resampled to the MNI space according to the fsaverage template. Then with the help of the glm-fit script of Freesurfer, the data were fitted according to the general linear model and the thickness of the structural maps was analyzed. In the end, the inflated map of the group analysis was prepared. Statistical analysis for group differences was performed using R programming using RStudio5 version 1.2.1335 on Mac. The t-test analyses were applied to our data for significance at p < .05. Pearson product-moment correlation coefficient test was used to test whether a correlation was present between disease duration and changes in areas with significant cortical thickness and volumes.
Results
There was no significant difference in terms of gender and age between the patients and HCs (t-test, p: 0.13 and 0.15, respectively).Structural Changes:
There was no significant volume change in the hippocampi and thalami in comparison of the patients vs. HCs (p ≥ .05). Comparison of the right vs left hippocampus and thalamus did not yield any difference either (p ≥ .05). However, in the patients, both amygdala were greater than HCs (p for right .03, p for left .00009) with no difference between right and left amygdala of the patients (p ≥ .05) (Table 1). Amygdala size showed no relation with the duration of the epileptic seizures according to the Pearson product-moment correlation coefficient test.
In the patients, cortical thickness was increased extensively in the left cerebral hemisphere compared with the right side including the frontal lobe, opercula, temporal lobe, occipital lobes. The cortex was increased in the right hemisphere in fewer areas including the cingulate gyrus and entorhinal cortex (Table 2).
As compared with HCs, the patients showed significantly reduced thickness in the left insula (p < .003). On the other hand, there was a widespread significant increase in the cortical thickness in both hemispheres (Table 3, Figure 2). The patients had reductions in cortical thickness in a few areas: left temporal lobe (ipsilateral to TLM) and to a lesser extent right frontotemporal lobes. Figure 1 shows relative mean values of those significant areas in the patients vs. HCs. No correlation was found between the disease duration and cortical thickness in the patients.
Conclusion
Although LTM usually appears as a tiny and hardly detectable lesion on MRI, it is associated with extensive cortical abnormalities mostly thickening and limited thinning. Together with larger amygdala these findings may reflect changes due to repeated cortical activation or structural plasticity resulting from long-standing seizures triggered by LTM.Acknowledgements
No acknowledgement found.References
- Fischl, B., et al. (2002). "Automated Labeling of Neuroanatomical Structures in the Human Brain Neuron."
- Iglesias, J. E., et al. (2015). "A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI." Neuroimage 115: 117-137.
- Saygin, Z. M., et al. (2017). "High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas." Neuroimage 155: 370-382.
- Iglesias, J. E., et al. (2018). "A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology." Neuroimage 183: 314-326.
- RStudio Team (2018). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/.
Figures
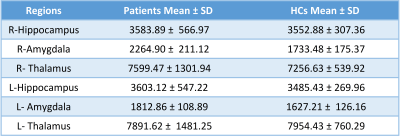
Table 1. Mean Volumes of the subcortical regions in patients vs. HCs.
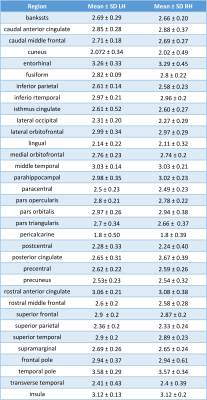
Table 2. Region’s cortical thickness measurements in patients
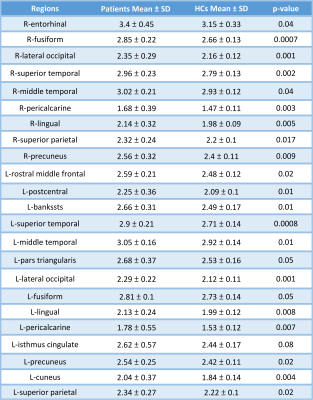
Table 3. Regions with significantly increased cortical thickness in the patients vs. HCs.
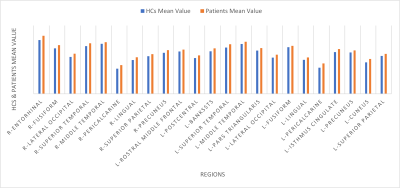
Figure 1. Cortical areas with statistically significant increased thickness in patients compared with HCs
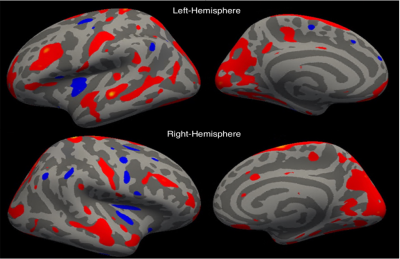
Figure 2. Cortical thickness in patients vs HC shown on the inflated surface of the brain. Red and blue show regions with significantly thicker cortex in the patients and HCs respectively.