1027
Multi-Excitation Actuator Design for Anisotropic Brain MRE1Biomedical Engineering, University of Delaware, Newark, DE, United States, 2Mechanical Engineering and Material Science, Washington University in St. Louis, St. Louis, MO, United States, 3Thayer School of Engineering, Dartmouth College, Hanover, NH, United States, 4Mechanical Engineering, Université de Sherbrooke, Sherbrooke, QC, Canada, 5Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States
Synopsis
The goal of this study is to thoroughly examine a novel customized multi-excitation MRE actuator combining both left-right (LR) and anterior-posterior (AP) drivers for use in estimating anisotropic mechanical properties of the brain. We demonstrate the key differences in wave patterns and how these lead to distinct stiffness estimations. The displacement fields from both excitations were combined to recover repeatable anisotropic properties in the corticospinal tract. The improved ME-MRE actuator can be used for future studies of white matter mechanical properties in health and disease.
Introduction
Magnetic resonance elastography (MRE) produces quantitative maps of tissue mechanical properties in the human brain.1 Mapping MRE outcomes in highly fibrous tissue, such as white matter, poses a challenge due to common assumptions of mechanical isotropy in inversion schemes; as such, MRE outcomes have shown a dependence on the direction of applied excitation, leading to differing property estimates in anisotropic tissue.2 White matter can be described with a nearly incompressible transversely isotropic (NITI) model but requires the generation of displacement fields with multiple propagation and polarization directions to solve for the complex shear modulus, tensile anisotropy, and shear anisotropy.3 Generating sufficient waves in the brain can be achieved with multi-excitation MRE (ME-MRE) that combines two passive drivers configured to apply vibrations in the anterior-posterior (AP) and the left-right (LR) directions.4 Here we propose a custom actuator, building upon a previous design5, to improve an approach to LR excitation that had in the past shown low repeatability. By affixing the driver to the head coil, this design was expected to improve functionality and data quality relative to previous experiments.2,4 The purpose of this work is to thoroughly characterize the stability of the novel actuator and demonstrate the capability to acquire high quality data for repeatable estimation of isotropic and anisotropic mechanical properties in white matter tracts.Methods
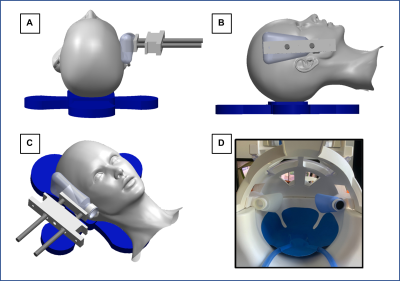
Actuator Design: Excitations are generated by an active pneumatic driver (Resoundant) with two passive drivers (Fig 1A-C): the standard pillow driver for AP excitation, and a lateral custom driver attached to the MRI head coil for LR excitation. The custom LR set up includes two flexible bottles attached to the head coil via custom 3D printed pieces that include adjustability to fit variable head size (Fig 1D). Either lateral driver can be active while the opposite driver stabilizes the head.Imaging Protocol: Two MRE scans were acquired separately for LR and AP excitations at 50 Hz. A 3D multiband, multishot spiral MRE sequence6 was used to image whole-brain displacements at 2 mm isotropic resolution (240x240x128 mm FOV, 64 slices, TR/TE = 2240/76 ms). Auxiliary scans included diffusion tensor imaging (DTI) with resolution and FOV matched to MRE to estimate fiber direction, and T1-weighted MPRAGE at 0.9 mm resolution to localize tracts.
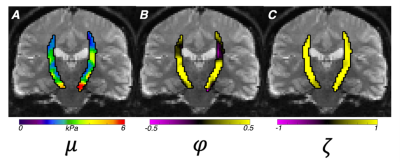
Performance Analysis: To characterize performance of the ME-MRE actuator, a single healthy subject (M, 23y) completed ten repetitions of the protocol on a 3T Siemens Prisma scanner using a 20-channel head coil. We determined wave propagation and polarization directions for both AP and LR excitation.7 To determine data quality we calculated OSS-SNR8 for each dataset, and bulk intrascan 3D translational and rotational motion for each excitation were estimated to assess the stability of our custom device using MCFLIRT in FSL.9 Shear wave fields from each excitation were inverted separately using the standard isotropic NLI10 to estimate the complex shear modulus, and the same fields were used together with fiber direction from DTI in a transversely isotropic NLI (TI-NLI)11,12 to estimate shear modulus, μ, shear anisotropy, φ (μ1/μ2-1), and tensile anisotropy, ζ (E1/E2-1). The anisotropic mechanical properties were examined in the corticospinal tract (CST) with mask determined via registration from the JHU white matter atlas.13
Results and Discussion
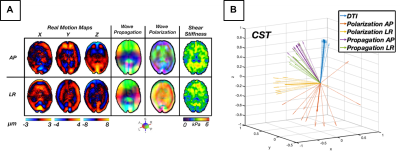
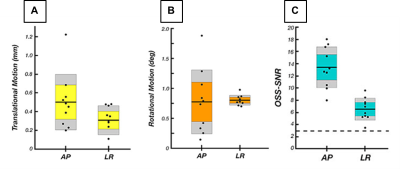
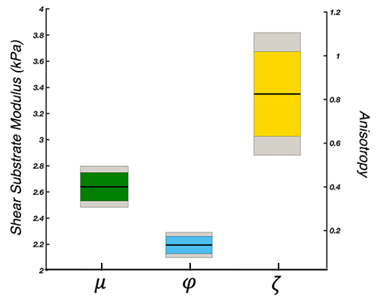
For our novel LR excitation driver, we evaluated wave characteristics, which revealed similar propagation direction and stiffness patterns in comparison to AP excitation, whereas the polarization directions were visibly different (Figure 2A). This ultimately resulted in different shear stiffness estimates when used in isotropic inversion, as seen previously2, which is presumably due to different slow and fast wave content in the displacement fields. Figure 2B illustrates how propagation directions are similar between AP and LR within the CST while polarization direction differs, and that wave characteristics are repeatable across datasets. Analysis of intrascan motion was used to determine how much a participant moves during a scan which could signal driver instability. Data from the LR driver exhibited lower overall rotational and translational motion than the AP driver (Figure 3A), which is known to produce high quality data14, so we can conclude that the new design is stable. LR excitation provides lower OSS-SNR values than AP, but all repetitions are above the threshold of 3.0 (Figure 3B), and this can likely be addressed through driver amplitude. We used TI-NLI to estimate anisotropic properties from both AP and LR motion fields. Figure 4 shows example property estimates within the CST. In Figure 5 anisotropic analysis of repeated scans showed very repeatable estimates of μ=2.64±0.16 (kPa), with repeatability consistent with previous isotropic results. The two anisotropy measures φ=0.13±0.06 and ζ=0.83±0.28 were more variable but still consistently positive, as expected. Uncertainty in recovered properties could be explained, in part, by misregistration or uncertainty in fiber directions from DTI, which will be investigated in future work.Conclusion
This study investigated a novel ME-MRE actuator to generate high quality displacement fields for repeatable anisotropic inversion. With improvements to ME-MRE actuator stability and repeatability, our new protocol will serve to measure the anisotropy of white matter tracts for use in studying white matter health in neurological conditions.Acknowledgements
NIH R01-EB027577References
1. Hiscox L V, Johnson CL, Barnhill E, et al. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys Med Biol. 2016;61(24):R401-R437. doi:10.1088/0031-9155/61/24/R401
2. Anderson AT, Van Houten EEW, McGarry MDJ, et al. Observation of direction-dependent mechanical properties in the human brain with multi-excitation MR elastography. J Mech Behav Biomed Mater. 2016; 59:538-546. doi: 10.1016/j.jmbbm.2016.03.005
3. Tweten DJ, Okamoto RJ, Bayly P V. Requirements for accurate estimation of anisotropic material parameters by magnetic resonance elastography: A computational study. Magn Reson Med. 2017;78(6):2360-2372. doi:10.1002/mrm.26600 4. Smith D, Guertler CA, Okamoto RJ, Romano A, Bayly P V, Johnson CL. Multi-Excitation MR Elastography of the Brain: Wave Propagation in Anisotropic White Matter. J Biomech Eng. 2020;142(7):710051-710059. doi:10.1115/1.4046199
5. Kailash K, Rifkin J, Ireland J, Okamoto RJ, Bayly P V. Design and Evaluation of a Lateral Head Excitation Device for MR Elastography of the Brain. Biomedical Engineering Society Annual Meeting 2019 Philadelphia, Pennsylvania Oct 16-19, 2019.
6. Johnson CL, Holtrop JL, Anderson AT, Sutton BP. Brain MR Elastography with Multiband Excitation and Nonlinear Motion-Induced Phase Error Correction. 24th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Singapore, May 7-13, 2016, p. 1951.
7. Okamoto RJ, Romano AJ, Johnson CL, Bayly P V. Insights Into Traumatic Brain Injury From MRI of Harmonic Brain Motion. J Exp Neurosci. 2019; 13:1179069519840444. doi:10.1177/1179069519840444
8. McGarry MDJ, Van Houten EEW, Perriñez PR, Pattison AJ, Weaver JB, Paulsen KD. An octahedral shear strain-based measure of SNR for 3D MR elastography. Phys Med Biol. 2011;56(13):N153-64. doi:10.1088/0031-9155/56/13/N02
9. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825-841. doi:10.1016/s1053-8119(02)91132-8
10. McGarry MDJ, Van Houten EEW, Johnson CL, et al. Multiresolution MR elastography using nonlinear inversion. Med Phys. 2012;39(10):6388-6396. doi:10.1118/1.4754649
11. McGarry MDJ, Van Houten E, Guertler C, et al. A heterogenous, time harmonic, nearly incompressible transverse isotropic finite element brain simulation platform for MR elastography. Phys Med Biol. Published online June 2020. doi:10.1088/1361-6560/ab9a84
12. McGarry MDJ, Van Houten EEW, Sowinski DR, et al. Model-Based Heterogeneous Transverse Isotropic MR Elastography Inversion for Brain Tissue with Aligned Fiber Tracts. 28th Annual Meeting of the International Society for Magnetic Resonance in Medicine
13. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39(1):336-347.
14. Murphy MC, Huston J 3rd, Jack CRJ, et al. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS One. 2013;8(12): e81668. doi: 10.1371/journal.pone.0081668
Figures