1016
A viscoelastic phantom of the healthy human liver for elastography in MRI and ultrasound1Charité - Universitätsmedizin Berlin, Berlin, Germany
Synopsis
Elastography in MRI (MRE) and ultrasound (USE) are the most reliable imaging techniques for liver fibrosis detection. However, there is no established, standardized material that mimics the elasticity and viscous behavior of liver tissue and can be used as a phantom for MRE and USE. We developed a phantom based on polyacrylamide which shows a viscoelastic dispersion from 5-3000 Hz similar to that observed in the healthy human liver, which is measurable by MRE and USE, stable over months and can be produced in a reproducible way. The novel phantom facilitates technical improvements and cross-modality comparisons in MRE and USE.
Introduction
Experiments on standardized and realistic viscoelastic phantom materials are crucial for technical development of elastography methods, improving data consistency and clinical acceptance. However, current commercial phantoms (Elasticity QA Phantom 049, CIRS, Inc. USA and MR Elastography Phantom, Resoundant, Inc. USA) cannot mimic realistic tissue properties in terms of shear stiffness and viscous damping of shear waves [1]. Literature data show that healthy human liver has a marked shear wave speed (SWS) dispersion which is not mimicked by available phantoms [2-8]. Especially ultrasound elastography (USE) suffers from the fact that methods by different vendors provide different SWS values. Quantitative Imaging Biomarkers Alliance (QIBA, RSNA) has addressed this problem and recommended using phantoms with realistic viscoelastic properties [9, 10]. Furthermore, available phantoms are not well investigable by both magnetic resonance elastography (MRE) and USE. We here address the need for a well-characterized phantom which mimics viscoelastic properties of liver tissue, can be standardized manufactured and is be suitable for MRE and USE.Methods
Phantom preparationThree phantoms were manufactured consisting of $$$74.1\,\mathrm{wt}\%$$$ water, $$$22\,\mathrm{wt}\%$$$ acrylamide, $$$1.7\,\mathrm{wt}\%$$$ ammonium persulfate and $$$1.7\,\mathrm{wt}\%$$$ tetramethylethylendiamin. Microcrystalline cellulose ($$$0.5\,\mathrm{wt}\%$$$) was added providing scatters for ultrasound. The material was filled into a two-liter cuboid container ($$$14.5\,\mathrm{cm}\,\times\,14.5\,\mathrm{cm}\,\times\,9.5\,\mathrm{cm}$$$) and stored at room temperature.
Viscoelastic characterization
After one month ripening, one phantom was fully characterized over a wide frequency range ($$$5\,-\,3000\,\mathrm{Hz}$$$) using rheomtery, MRE (clinical MRI and compact tabletop MRI scanner) and USE. For each vibration frequency, SWS as surrogate for elastic properties and penetration rate (PR) as a surrogate for viscous properties were derived. The relation to the complex shear modulus $$$G^*=|G^*|\cdot\exp(\mathrm{i}\,\phi)$$$ is: $$SWS=\sqrt{\frac{2\,|G^*|}{\rho\,(1+\cos(\phi))}}$$ $$PR=\sqrt{\frac{2\,|G^*|}{\rho\,(1-\cos(\phi))}}\frac{1}{2\,\pi}$$ Further imaging parameters of MRI (T1, T2, apparent diffusion coefficient (ADC)) and ultrasound (ultrasound speed, ultrasound attenuation and echogenicity) were obtained.
Rheomtery
Viscoelastic properties in the low frequency range ($$$5\,–\,60\,\mathrm{Hz}$$$) were quantified using shear oscillatory rheomtery (Modular Compact Rheometer 301, Anton Paar GmbH, Austria).
Clinical MRE and USE
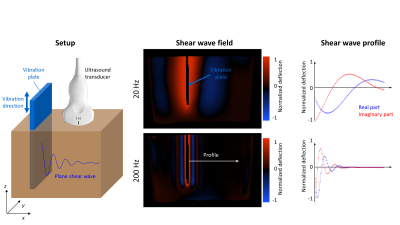
A vibration plate was immersed in the phantom material in order to induce plane shear waves in the frequency range from $$$20$$$ to $$$200\,\mathrm{Hz}$$$ (Figure 1). For clinical MRE, experiments were performed on a 3-Tesla MRI scanner (Siemens Magnetom Lumina, Erlangen, Germany) using a single-shot, spin-echo echo-planar imaging sequence. Further imaging parameters were: echo time $$$(TE)\,=\,112\,\mathrm{ms}$$$, repetition time $$$(TR)\,=\,2000\,\mathrm{ms}$$$, motion encoding gradient (MEG) amplitude$$$\,=\,34\,\mathrm{mT}/\mathrm{m}$$$, MEG duration$$$\,=\,47\,\mathrm{ms}$$$ with fractional encoding. For USE, we used a SonixMD (Utrasonix, USA) scanner with a convex probe (C5-2) and a frame rate of $$$125\,\mathrm{Hz}$$$ [6]. Analysis for each frequency was done by drawing manual profiles perpendicular to the wave fronts and fitting by the analytical solution of a plane shear wave.
Tabletop MRE
For high frequency characterization ($$$300\,-\,3000\,\mathrm{Hz}$$$) a customized 0.5-Tesla tabletop MRI scanner (Mag Spec, Pure Devices GmbH, Germany) with a permanent magnet was used [11]. The phantom was filled in a glass tube ($$$7\,\mathrm{mm}$$$ diameter) and sealed. Due to the cylindrical geometry, the shear wave field could be described and analyzed by analytical Bessel function. A spin-echo imaging sequence was used with the following parameters: $$$TE\,=\,27\,\mathrm{ms}$$$, $$$TR\,=\,1100\,\mathrm{ms}$$$, MEG amplitude$$$\,=\,200\,\mathrm{mT}/\mathrm{m}$$$, MEG duration$$$\,=\,20\,\mathrm{ms}$$$. Diffusion weighted imaging (DWI) was used with $$$7\,b$$$-factors, linearly spaced between $$$50\,-\,800\,\mathrm{s}/\mathrm{mm}^2$$$ to compute ADC.
Long-term stability and reproducibility
All three phantoms were characterized after one month ripening by tabletop MRE and rheomtery ensuring reproducibility. One phantom was measured over four months by tabletop MRE assessing long-term stability.
Results
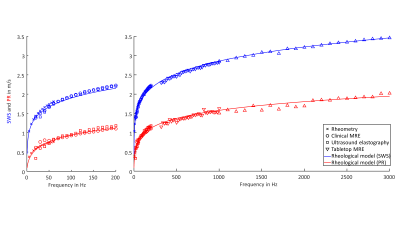
SWS and PR values measured by Rheometry, clinical and tabletop MRE and USE were consistent over a wide frequency range (Figure 2). SWS and PR followed the fractional Maxwell-model (Springpot serial to dashpot; $$$\alpha_{\mathrm{SP}}\,=\,0.345\,\pm\,0.002$$$, $$$\mu_{\mathrm{SP}}\,=\,8.45\,\pm\,0.04\,\mathrm{kPa}$$$, $$$\eta_{\mathrm{SP}}$$$ set to $$$1\,\mathrm{Pa}\,\mathrm{s}$$$ and $$$\eta_{\mathrm{DP}}\,=\,55\,\pm\,5\,\mathrm{Pa}\,\mathrm{s}$$$). For reproducibility, we calculated for each frequency the range between the minimum and maximum values of SWS and PR over all three phantoms which was, on average, $$$0.07\,\mathrm{m}/\mathrm{s}$$$ for the two parameters. For the long-term behavior, we observed a significant increase of SWS ($$$0.14\,\pm\,0.06\,\mathrm{m}/\mathrm{s}$$$, $$$p\,=\,7\cdot10^{-13}$$$) and penetration rate ($$$0.25\,\pm\,0.09\,\mathrm{m}/\mathrm{s}$$$, $$$p\,=\,2\cdot10^{-14}$$$) during the first month. From one to four months we observed a further weak but significant increase of SWS ($$$0.05\,\pm\,0.06$$$, $$$p\,=\,9\cdot10^{-4}$$$) and of PR ($$$0.07\,\pm\,0.10$$$, $$$p\,=\,7\cdot10^{-4}$$$). Parameters measured by tabletop MRI at $$$0.5\,\mathrm{T}$$$ were $$$T1\,=\,923\,\pm\,191\,\mathrm{ms}$$$, $$$T2\,=\,686\,\pm\,9\,\mathrm{ms}$$$ and $$$ADC\,=\,1439\,\pm\,149\,\mathrm{µm}^2/\mathrm{s}$$$. Parameters derived by ultrasound were speed of sound$$$\,=\,1606\,\pm\,8\,\mathrm{m}/\mathrm{s}$$$ and ultrasound attenuation$$$\,=\,0.4\,\pm\,0.01\,\mathrm{dB}/\mathrm{cm}/\mathrm{MHz}$$$. Echogenicity was visually similar to that of healthy human liver.Discussion
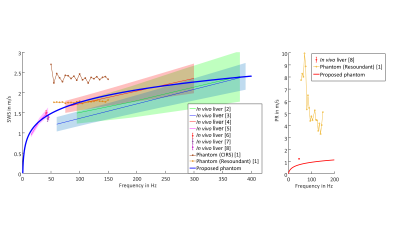
Our proposed phantom shows SWS dispersion very similar to in vivo healthy human liver (Figure 3). Furthermore, the viscosity quantified by PR better reflects the behavior of in vivo human liver than other phantoms, which have a higher penetration rate [8]. The good reproducibility of the phantom material properties permits a straightforward comparison of techniques in MRE and USE across modalities and platforms. Over time we observed a continuous decreasing change of SWS and PR, indicating that the phantom becomes stable over time. However, the long-term stability needs to be further investigated. MRI and ultrasound parameters are roughly comparable to in vivo liver.Conclusion
We have introduced a novel phantom for MRE and USE which has viscoelastic properties close to healthy human liver within a wide frequency range. The phantom is measurable by MRI and ultrasound and can thus be used for a straightforward comparison of elastography techniques across modalities and platforms.Acknowledgements
Funding from the German Research Foundation (GRK 2260 BIOQIC, SFB1340 Matrix in Vision) is gratefully acknowledged.References
1. Felix Schrank, H.T., Angela Ariza de Schellenberger, Jürgen Braun, and I. Sack, Heparin as MRE phantom material with viscoelastic powerlaw properties similar to soft biological tissues. 2017, Charité-Universitätsmedizin Berlin, Berlin, Germany
2. Chen, S., et al., Assessment of liver viscoelasticity by using shear waves induced by ultrasound radiation force. Radiology, 2013. 266(3): p. 964-70.
3. Muller, M., et al., Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol, 2009. 35(2): p. 219-29.
4. Nightingale, K.R., et al., Derivation and analysis of viscoelastic properties in human liver: impact of frequency on fibrosis and steatosis staging. IEEE Trans Ultrason Ferroelectr Freq Control, 2015. 62(1): p. 165-75.
5. Dittmann, F., et al., In vivo wideband multifrequency MR elastography of the human brain and liver. Magn Reson Med, 2016. 76(4): p. 1116-26.
6. Tzschätzsch, H., et al., Two-Dimensional Time-Harmonic Elastography of the Human Liver and Spleen. Ultrasound Med Biol, 2016. 42(11): p. 2562-2571.
7. Tzschätzsch, H., et al., Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal, 2016. 30: p. 1-10.
8. Hudert, C.A., et al., Tomoelastography for the Evaluation of Pediatric Nonalcoholic Fatty Liver Disease. Investigative radiology, 2019. 54(4): p. 198-203.
9. Hall, T.J., et al. RSNA/QIBA: Shear wave speed as a biomarker for liver fibrosis staging. in 2013 IEEE International Ultrasonics Symposium (IUS). 2013.
10. Palmeri, M., et al. RSNA QIBA ultrasound shear wave speed Phase II phantom study in viscoelastic media. in 2015 IEEE International Ultrasonics Symposium (IUS). 2015.
11. Braun, J., et al., A compact 0.5 T MR elastography device and its application for studying viscoelasticity changes in biological tissues during progressive formalin fixation. Magnetic resonance in medicine, 2018. 79(1): p. 470-478.
Figures