1009
3D-MRE as independent predictor of direct portal venous pressure compared to Biopsy derived Fibrosis Measurements1LVTS, INSERM U1148, Paris, France, 2Department of Biomedical Engineering, King's College London, London, United Kingdom, 3Biomedical and Metabolic Imaging Branch, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, MD, United States, 4Center for Cancer Research, National Cancer Institute, Bethesda, MD, United States, 5Department of Gastroenterology and Liver Diseases, Ben-Gurion University of the Negev, Be'er Sheva, Israel, 6Department of Internal Medicine, Duke University, Durham, NC, United States
Synopsis
The assessment of liver fibrosis and inflammation is crucial to the clinical management of patients suffering from liver diseases. Portal venous pressure assessment has important prognostic implications for hepatic disease patients’ care. However, it can only be measured invasively. In this work we show that liver stiffness quantified via 3DMRE is a strong independent predictor of DPVP compared to invasive biopsy. Moreover stiffness as evaluated with 3DMRE is an operator independent and non-invasive biomarker which could hence constitute an optimal method for diagnosis and fibrosis staging in all patients and a non-invasive surrogate of DPVP.
Introduction
The assessment of liver fibrosis and inflammation is crucial to the clinical management of patients suffering from liver diseases. Portal venous pressure assessment has important prognostic implications for hepatic disease patients’ care as it is the result of an increased hepatic vascular resistance and portal inflow1. Most commonly the increased resistance to portal blood flow is due to chronic hepatitis, the most common cause of portal hypertension worldwide, and hepatic schistosomiasis2. However, its assessment can only be achieved invasively through direct portal vein pressure (DPVP) or hepatic vein pressure gradient (HVPG) measurements. Among non-invasive biomarkers of liver diseases, liver stiffness quantified via Magnetic Resonance Elastography (3DMRE) has already proven to be an optimal parameter to stage hepatic fibrosis in the clinical setting3. The purpose of this study is to evaluate the diagnostic value of 3DMRE measured liver stiffness, independent from liver fibrosis, as a non-invasive measure of DPVP.Methods
A total of 29 patients with hepatitis C participated to this prospective study, with average age of 57.6 ± 9.7 years, BMI 27.5 ± 4.0 kg m-2 and 18 where men. DPVP was measured via direct cannulation of the portal vein. All patients had trans-jugular liver biopsy for quantifying degree of fibrosis (ISHAK score) and 3DMRE performed during routine liver MRI at 3T. A surface mechanical transducer (Philips Healthcare, Hamburg, Germany) applied to the right upper abdomen was used to generate the vibration with a frequency of 56 Hz. The MRE sequence was a modified 2D multi-slice gradient-recalled echo sequence with an in-phase TE=6.9ms4. The waves were imaged in 3D within a volume centered transversally in the middle of the liver, with 4mm isotropic resolution, acquiring 3 spatial motion directions plus one reference scan during breath-holds of 14s each. The MRE data were processed in Fourier Space to remove high frequency noise using a low pass Blackmann Harris filter and were subsequently reconstructed as in Sinkus et al.5. This allowed to calculate the complex shear modulus G*=G’ +iG’’, where G’(Gd) is the shear stiffness or dynamic modulus and G’’(Gl) is the shear viscosity or loss modulus. The biomechanical parameters were then compared with the DPVP and the fibrosis score evaluated via histology. Spearman correlation and least squares multiple regression were performed to identify the relations between these variables. Finally, receiver operator curves (ROC) were generated to evaluate the diagnostic performance of MRE measured stiffness vs Ishak histology to distinguish patients with clinically significant portal hypertension (DPVP>11mmHg) (CSPH).Results and discussion
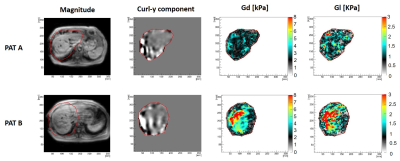
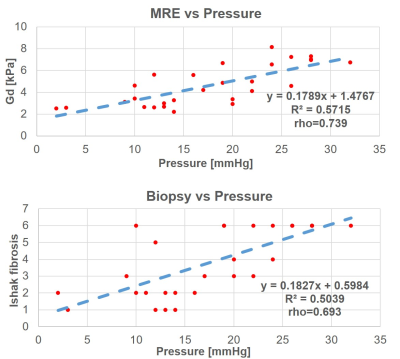
Figure 1 shows anatomy, y-component of the curl of the displacement vector, shear stiffness and viscosity, for two selected patients with low (Pat A) and high DPVP (Pat B) (Pat A: DPVP 3mmHg and fibrosis score 1, Pat B: DPVP 28 mmHg and fibrosis score 6). Figure 2 shows the correlation curves of Gd and Ishak fibrosis score vs DPVP. A better correlation was found for the MRE pressure surrogate. Both hepatic fibrosis and 3DMRE stiffness showed statistically significant (p<0.0001) positive correlation with DPVP. However, in stepwise multilinear regression (including variance inflation factor) analysis, 3D-MRE stiffness was the only independent predictor of DPVP (P<0.0001). Moreover, ROC analysis showed that out of the two ONLY 3DMRE has a statistically significant (p<0.0008) area under the curve (AUC) of 80.3% for identifying patients with CSPH.These results could be explained by the subjective nature of histology results that are highly susceptible to sampling errors related to the small sample size and the heterogeneity in degree of fibrosis, and variability of pathologist interpretation6. On the contrary, the assessment of liver stiffness via 3DMRE provides an objective comprehensive assessment of the hepatic condition, which explains the better correlation of this biomarker with the invasive gold standard DPVP.
Conclusions
Stiffness as quantified by non-invasive 3DMRE is a strong independent predictor of DPVP compared to invasive biopsy. Contrarily to fibrosis score, stiffness as evaluated with 3DMRE is an operator independent and non-invasive biomarker which could hence constitute an optimal method for diagnosis and fibrosis staging in all patients. Moreover, it could be used as a non-invasive surrogate of DPVP to identify patients with portal hypertension for early diagnosis and close therapeutic monitoring.Acknowledgements
This project has received funding from National Institute of Health (NIH): grant number 1R01EB028664-01.References
1. Mahl, T.C., et al. (1990). Pathophysiology of portal hypertension and variceal bleeding. . Surg. Clin. North. Am. , 70: 251 – 66.
2. Berzigotti, A., et al. (2013). Assessing portal hypertension in liver diseases. Expert review of gastroenterology & hepatology, 7(2), 141-155.
3. Talwalkar, J.A., et al. (2008). Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology, 47:332–342.
4. Garteiser, P., et al. (2013). Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR Biomed., 26:1326–1335.
5. Sinkus, R., et al. (2018). Rheological determinants for simultaneous staging of hepatic fibrosis and inflammation in patients with chronic liver disease. NMR in Biomedicine, 31(10), e3956.
6. Ratziu, V., et al. (2005). Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology, 128(7):1898–1906.
Figures