1003
Heterogeneity of Viscoelastic Properties in Prostate Cancer Assessed by Ex Vivo MR Elastography1Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Pathology, University of Illinois at Chicago, Chicago, IL, United States, 4Urology, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
We aimed to evaluate the heterogeneity of viscoelastic tissue properties of fresh prostatectomy specimens of men with prostate cancer using MR elastography and histopathology as a reference. Our results suggest that prostate cancer is characterized by a stiff yet homogeneous biomechanical signature. A possible explanation for this finding might be differences of healthy vs. cancerous tissue based on anatomical zones and their specific nonmalignant pathologies (prostatitis in the peripheral zone and benign prostatic hyperplasia in the central gland), and the unique nondestructive growth pattern of PC with intervening stroma which might provide a rigid scaffold.
Introduction
We aimed to evaluate the heterogeneity of viscoelastic tissue properties of fresh prostatectomy specimens of men with prostate cancer (PC) using MR elastography (MRE) and histopathology as a reference. Despite the success of in vivo multiparametric MRI for PC detection, moderate inter- and intraobserver agreement and low specificity constitute significant limitations.1 In contrast to most solid tumors, PC is often multifocal and characterized by a nondestructive growth pattern with intervening stroma between benign glands and irregular tongues of tumor tissue, which can extend far from the main tumor.2 This unique pattern of spatial and morphological variability in PC complicates diagnostics and therapy planning and made PC the only cancer to be diagnosed by systematic 12-core randomized biopsies.2Methods
This explorative study is a secondary analysis of prospectively collected data at a single institution.3 The study was approved by the local IRB and written informed consent was obtained from all men who had a mean age of 62 ± 8 years. A total of 12 prostate specimens without prior radiation therapy were included. Fresh prostatectomy specimens without formalin fixation were scanned directly after surgery using a preclinical 9.4T MRI scanner (310/ASR, Agilent, Santa Clara, California). A piezoelectric actuator (P-840.1, Physik Instrumente, Karlsruhe, Germany) generated shear waves at 500 Hz (a single specimen was investigated at 250 Hz due to its attenuation properties) and a spin-echo MRE sequence based on sample interval modulation was used to simultaneously acquire wave field displacements along the x-, y- and z-direction.4,5 Maps of the magnitude of the complex shear modulus (|G*| in kPa) were calculated with its real part (G’ in kPa) and its imaginary part (G’’ in kPa) representing storage and loss modulus, respectively.5,6 Prostates were divided in 12 segments for a segment-based assessment of tissue properties and histopathology. The coefficient of variation (CV in %) was determined for the segment-based quantification of heterogeneity of |G*|, G’ and G’’.Results
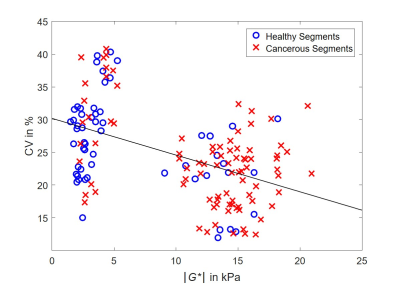
Mean values of cancerous segments were significantly increased compared to healthy segments: |G*| with 12.13 kPa vs. 6.14 kPa, G’ with 10.84 kPa vs. 5.44 kPa and G’’ with 5.45 kPa vs. 2.92 kPa, all p < 0.001.3 On the contrary, CV values were significantly increased for healthy segments: CV of |G*| with 23.59 % vs. 26.32 % (p = 0.014), CV of G’ with 27.05 % vs. 37.84 % (p < 0.001) and CV of G’’ with 36.51 % vs. 50.37 % (p = 0.008). Pearson's correlation coefficient showed a significant moderate negative correlation between CV and mean values of |G*| (r = -0.48) (figure 1), G’ (r = -0.46) and G’’ (r = -0.34), all with p ≤ 0.001. Diagnostic performance of mean values was good to excellent (AUC of mean |G*|, G’ and G’’ = 0.80, 0.81, 0.78, respectively) whereas performance of CV values was moderate (AUC of CV of |G*|, G’ and G’’ = 0.62, 0.65, 0.63, respectively).Discussion
We aimed to evaluate the heterogeneity of viscoelastic tissue properties of fresh prostatectomy specimens of men with PC using MRE and histopathology as a reference. Two key observations were made: i) A statistically significant increased CV of |G*|, G’ and G’’ was found in healthy segments as compared to cancerous segments, which indicates reduced mechanical property heterogeneity in PC. ii) Diagnostic performance of CV did not exceed performance of mean values.As previously reported, viscoelastic properties increase with PC.3,7–10 Different anatomical zones combined with their specific nonmalignant pathologies, such as prostatitis in the peripheral zone and benign prostatic hyperplasia in the central gland, might possibly be responsible for increased heterogeneity in healthy segments. For cancerous segments in turn, the far-reaching nondestructive growth pattern with intervening stroma increases stiffness but at the same time reduces heterogeneity, possibly by providing a rigid scaffold in the affected segment. McGrath et al investigated prostatectomy specimens of men with PC and prior radiation therapy (n = 4).7 They found highest stiffness values for the peripheral zone (99 kPa), followed by PC (85 kPa) and with lowest values for the central gland (48 kPa). They observed a significant difference between the peripheral zone and central gland (p < 0.02) and central gland and PC (p < 0.03) but not for peripheral zone and PC. In agreement with our results, their findings emphasize the importance of anatomical zones and their specific nonmalignant pathologies for the biomechanical detection of PC. Although encouraging, our results need to be confirmed in a larger in vivo cohort.
In conclusion, our results suggest that PC is characterized by a stiff yet homogeneous biomechanical signature. A possible explanation for this finding might be differences of healthy vs. cancerous segments based on anatomical zones and their specific nonmalignant pathologies, and the unique nondestructive growth pattern of PC with intervening stroma which might provide a rigid scaffold in the affected area.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): RE 4161/1-1, RE 4161/1-2, RE 4161/2-1 (Rolf Reiter); SFB 1340 Matrix in Vision, project number 372486779 (Rolf Reiter); and GRK 2260 BIOQIC.References
1. Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019;76(3):340-351.
2. Tolkach Y, Kristiansen G. The Heterogeneity of Prostate Cancer: A Practical Approach. Pathobiology. 2018;85(1-2):108-116.
3. Reiter R, Majumdar S, Kearney S, et al. Prostate cancer assessment using MR elastography of fresh prostatectomy specimens at 9.4 T. Magn Reson Med. 2020;84(1):396-404.
4. Klatt D, Yasar TK, Royston TJ, Magin RL. Sample interval modulation for the simultaneous acquisition of displacement vector data in magnetic resonance elastography: theory and application. Phys Med Biol. 2013;58(24):8663-8675.
5. Klatt D, Johnson CL, Magin RL. Simultaneous, multidirectional acquisition of displacement fields in magnetic resonance elastography of the in vivo human brain. J Magn Reson Imaging. 2015;42(2):297-304.
6. Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5(4):237-254.
7. McGrath DM, Lee J, Foltz WD, et al. MR elastography to measure the effects of cancer and pathology fixation on prostate biomechanics, and comparison with T1, T2 and ADC. Phys Med Biol. 2017;62(3):1126-1148.
8. Sahebjavaher RS, Nir G, Gagnon LO, et al. MR elastography and diffusion-weighted imaging of ex vivo prostate cancer: Quantitative comparison to histopathology. NMR Biomed. 2015;28(1):89-100.
9. Dresner MA, Cheville JC, Myers RP, Ehman RL. MR Elastography of Prostate Cancer. Proc Intl Soc Mag Reson Med. 2003:578.
10. Asbach P, Ro S, Aldoj N, et al. In Vivo Quantification of Water Diffusion, Stiffness, and Tissue Fluidity in Benign Prostatic Hyperplasia and Prostate Cancer. Invest Radiol. 2020;55(8):524-530.