0992
Free-Breathing High-Resolution Quantitative First-Pass Perfusion Cardiac MR using Dual-Echo Dixon1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom, 3Magnetic Resonance Systems Lab, Delft University of Technology, Delft, Netherlands, 4Philips Healthcare Iberia, Madrid, Spain, 5Philips Research, Hamburg, Germany, 6MR Clinical Science, Philips Healthcare, Best, Netherlands, 7Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 8Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden, 9Center for Medical Image Science and Visualization (CMIV), Linköping University, Linköping, Sweden
Synopsis
First-pass perfusion cardiac MR (FP-CMR) is an essential tool for characterizing ischemic heart disease. Moreover, automated quantitative methods enable a reliable and operator-independent assessment of myocardial blood flow. Conventional FP-CMR has limited spatial resolution and is performed under breath-hold. Therefore, diagnostic accuracy is compromised by respiratory induced motion artifacts and false-positives due to dark-rim artifacts. To overcome these challenges, free-breathing Fat-water separation for mOtion-corrected Spatio-TEmporally accelerated myocardial peRfuSion (FOSTERS) has been proposed. Here, we implement and evaluate a high-resolution dual-echo Dixon version of FOSTERS and compare its quantitative performance to standard-resolution FP-CMR in patients with suspected cardiovascular disease.
Introduction
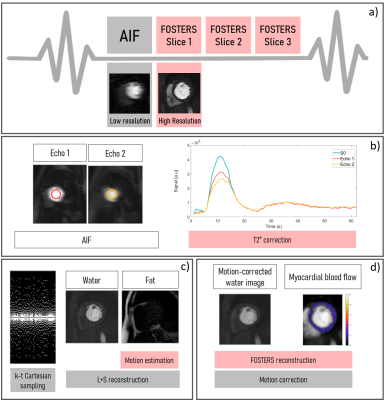
First-pass perfusion cardiac MR (FP-CMR) enables the non-invasive detection of ischemic heart disease. Conventional FP-CMR is performed under breath-hold to reduce respiratory motion, which can be challenging for patients.1,2 Moreover, low-resolution FP-CMR images are prone to dark-rim artifacts, which mimic perfusion defects and affect diagnostic accuracy. Pixel-wise quantification of myocardial blood flow (MBF) can be compromised by these artifacts and by signal loss caused by T2* decay at high contrast concentrations.1-4 Recently, the FOSTERS4,5 framework has been proposed to address respiratory motion, T2*-related signal loss (which causes MBF overestimation) and the limited spatial resolution. FOSTERS provides fat-only images, which are used to estimate respiratory motion, while motion-corrected water-only images are used for quantifying MBF. FOSTERS combines dynamically varying undersampling with a motion-corrected reconstruction using low-rank and sparsity constraints to achieve high-resolution FP-CMR images. The high-resolution acquisition is interleaved with a low-resolution image with low T1-sensitivity for estimating the arterial input function (AIF).6 Additionally, the multi-echo images are used for correcting the AIF for T2*-related signal losses to further improve MBF quantification. In this work, we propose an accelerated dual-echo version of FOSTERS to enable compatibility with stress perfusion. The performance of the free-breathing high-resolution dual-echo Dixon FP-CMR scheme is compared to the corresponding clinical low-resolution breath-hold FP-CMR in 8 patients with suspected cardiovascular disease.Methods
Eight patients (4 females, 36-78 years old) were scanned (ethically approved; informed written consent obtained) during rest with 8-fold k-t accelerated FOSTERS during the first-pass of a contrast bolus injection (0.075mmol/kg of Gadobutrol at 4ml/s followed by 25ml saline flush) on a 3T MRI scanner (Achieva, Philips, NL). FOSTERS consisted of an ECG-triggered dual-saturation, 2D dual-echo gradient echo sequence with the following parameters: FOV=320×300mm2, three short-axis slices (basal, mid, and apical); in-plane resolution=1.6×1.6mm2, slice thickness=10mm, TR/TE1/TE2=2.8/1.1/1.9ms, saturation delay=100ms, flip angle=15°, acquisition window=70ms and 54-87 dynamic frames. For comparison, patients were scanned with a clinical breath-hold low-resolution 2D FP-CMR acquisition6 with parameters: FOV=320×320mm2, three short-axis slices, in-plane resolution=2.6×2.6mm2, slice thickness=10mm, TR/TE=2.2/1ms, SENSE=2 and partial Fourier=0.75. To assess the motion correction performance of the dual-echo FOSTERS, an additional patient was scanned with FOSTERS in free-breathing and breath-hold, during the same CMR examination. The total acquisition time for all scans was 60s.The schematic of the free-breathing FOSTERS framework is shown in Fig. 1. Image reconstruction was implemented on the scanner with modified software and includes the following steps: 1) Dixon reconstruction with low-rank and sparsity constraints7 which generates water and fat images from k-t undersampled data after 5 iterations; 2) Fat-only images are used to estimate the frame-by-frame respiratory motion; 3) Translational motion correction is performed in k-space by applying a linear phase shift to the dual-echo images; 4) Motion-corrected water-only images are generated using a reconstruction with low-rank and sparsity constraints after 50 iterations. The echo images are used for estimating the AIF and T2*-related signal loss. Finally, FP-CMR images were automatically segmented using a deep learning-based method and MBF maps were generated using a Bayesian inference method.8 Dynamic images were non-rigidly motion corrected before quantification.
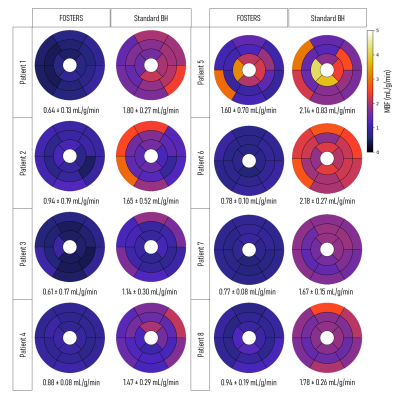
Results and Discussion
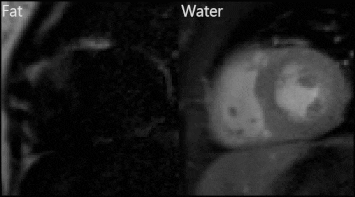
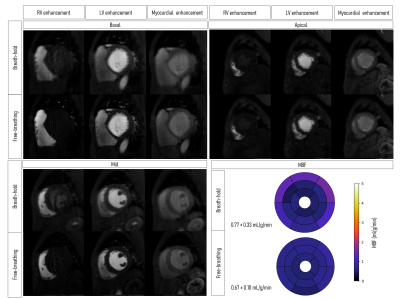
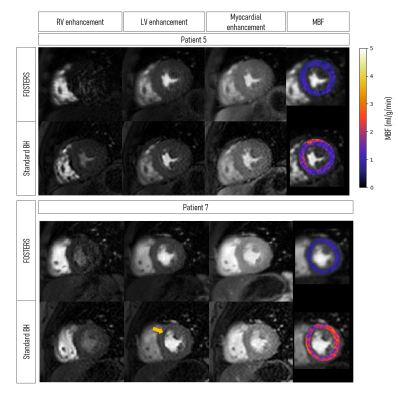
Representative fat and water dynamic images from one patient obtained using FOSTERS are shown in Fig. 2. The fat-only images contain enough structural information for estimating respiratory motion, to then generate motion-corrected diagnostic water-only images. Fig. 3 shows images from another patient where both free-breathing and breath-hold FOSTERS was acquired, to evaluate the motion correction performance. More uniform MBF maps were obtained using the free-breathing approach. This is likely because free-breathing acquisitions encourage more regular and shallower breathing, which is easier to correct, and reduces the risk for large amounts of motion that can occur at the start or end of a long breath-hold. Moreover, non-rigid motion correction performs better when correcting for small residual motion than in the presence of large motion. FOSTERS provides high-quality dynamic images with reduced dark-rim artefacts compared to the standard low-resolution FP-CMR images (Fig. 4). Moreover, FOSTERS provides uniform quantitative perfusion maps (Fig. 4-5), as expected in the absence of ischemia and scar under resting conditions, whereas the presence of motion introduces artifacts (larger standard deviation) in the standard low-resolution MBF maps. Patient 5 suffered from frequent ectopic heartbeats, and thus, cardiac motion is the likely cause of the inhomogeneous MBF maps. The mean MBF (± standard deviation, SD) values were 0.90 (± 0.31) and 1.72 (± 0.35) mL/min/g for FOSTERS and standard sequence, respectively. The corresponding mean (± SD) standard deviation of the MBF maps was 0.21 (± 0.21) and 0.36 (± 0.22) mL/min/g. Significant differences (p<0.05) in MBF were found between the two methods. This is probably in part due to the FOSTERS T2* correction of the AIF, and therefore more accurate MBF estimates, but also the denoising nature of the reconstruction, and smaller residual respiratory motion artifacts.Conclusion
FOSTERS, a k-t accelerated dual-saturation dual-echo Dixon FP-CMR framework, enables free-breathing and high-resolution FP-CMR and improves MBF quantification. Here, FOSTERS was compared to standard low-resolution breath-hold FP-CMR and provided higher quality diagnostic images with minimal dark-rim artifact. Furthermore, FOSTERS corrects for respiratory motion and T2*-related signal losses and hence, provides improved MBF maps. Future studies will aim to test FOSTERS in patients during stress.Acknowledgements
This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z] and The Swedish Research Council [grant 2018-04164].References
1. Kellman P, Arai AE. Imaging sequences for first pass perfusion - a review. J Cardiovasc Magn Reson. 2007;9(3):525-37.
2. Jerosh-Herold M. Quantification of myocardial perfusion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12(57):12-57.
3. Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19(1):43.
4. Scannell C, Correia T, Villa A, et al. Feasibility of free-breathing quantitative myocardial perfusion using multi-echo Dixon magnetic resonance imaging. Sci Rep. 2020;10:12684.
5. Tourais J, Schneider T, Scannell C, et al. High-Resolution Free-Breathing Quantitative Myocardial Perfusion MRI Using Multi-Echo Dixon. ISMRM 2020;1315.
6. Sanchez-Gonzalez J, Fernandez-Jimenez R, Nothnagel N, et al. Optimization of dual-saturation single bolus acquisition for quantitative cardiac perfusion and myocardial flow maps. J Cardiovasc Magn Reson. 2015; 7:21.
7. Otazo R, Kim D, Axel L, Sodickson D. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64(3):767-776.
8. Scannell C, Veta M, Villa A, et al. Deep‐Learning‐Based Preprocessing for Quantitative Myocardial Perfusion MRI. J Magn Reson Imaging. 2020;51:1689-1696.
Figures