0991
Cardiovascular MRI–derived myocardial strain vs. extracellular volume fractions in asymptomatic heart transplant patients1Department of Radiology, the Affiliated Hospital of Guizhou Medical University, Guiyang, China, China, 2Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, Wuhan , China, China, 3MR Collaboration, Siemens Healthineers Ltd., Shanghai, China, Shanghai, China, China, 4MR R&D, Siemens Medical Solutions Inc., Chicago, USA, Chicago, USA, IL, United States, 5Siemens Healthcare GmbH, Erlangen, Germany, Erlangen, Germany, Germany
Synopsis
Cardiac magnetic resonance imaging has excellent tissue resolution and can quantitatively evaluate myocardial deformations and tissue characteristics. In this study, heart transplant (HT) patients had changed myocardial strain (LCGLS, LVGCS, and LVGRS) and tissue parameters (T1, T2, and ECV). There were also moderate correlations between these two different measurements. We suggest that myocardial movement is related to myocardial edema and fibrosis, and myocardial strain measurements could be an alternative to ECV with gadolinium injections.
Introduction
Cardiac magnetic resonance (CMR)-derived myocardial strain and extracellular volume fractions (ECV) can reflect cardiac functional structure and tissue features, respectively. Myocardial strain measurements, including left ventricular (LV) longitudinal strain (LS), circumferential strain (CS) and radial strain (RS), are noninvasive methods to quantify myocardial deformation and recommend by adult guidelines for heart transplant (HT) patients to discover subclinical allograft dysfunction[1]. The CMR-derived ECV can be used as a noninvasive method to quantify myocardial interstitial volume, which seems to be fundamental to the process of adverse left ventricular remodeling in a range of heart diseases[2,3]. Recently, a few papers have shown the correlation between myocardial strain and ECV in asymptomatic HT patients[4]. This study aimed to explore the correlation between CMR-derived myocardial strain and ECV in these patients.Methods
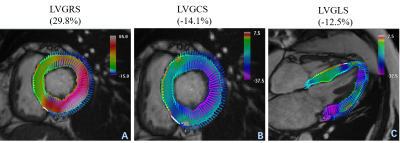
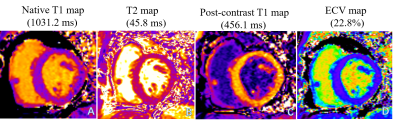
Between March and June 2017, 32 HT patients were referred for CMR scans at our Hospital. The exclusion criteria included reduced left ventricular ejection fractions (LVEF) (<50%), chronic atrial fibrillation, currently confirmed histologic or clinical indications of significant acute rejection, severe renal insufficiency, and contraindications of a CMR exam. 16 healthy volunteers were recruited. All subjects underwent standard CMR examinations with a 1.5 Tesla (T) MR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). A balanced steady-state free-precession sequence (bSSFP) was performed to acquire LV long-axis cine (including 2-, 3-, 4-chamber) images and a stack of short-axis cines. T1 mapping was performed on three standard LV short-axis slices (base, middle, and apex) before and 15 min after gadolinium injection. A prototypic T1-mapping sequence (Siemens Healthcare, Erlangen, Germany) was used for the pre- and post-contrast T1-map imaging and inline ECV map calculations. Specifically, a modified Look-Locker inversion recovery (MOLLI) sequence with a 5b(3b)3b sampling scheme and a 4b(1b)3b(1b)2b sampling scheme were used for the pre- and post-T1 maps. Patient hematocrit inputs obtained through blood sample analyses on the day of CMR examinations and during post-T1 mapping acquisitions (using the parameter card) can allow ECV maps to be automatically generated with the T1 maps. T2 mapping was acquired with three slices (identical to T1 mapping) before gadolinium injections using a T2-prepared single-shot bSSFP sequence. Strain values were analyzed using prototypic software (TrufiStrain, version 2.0, Siemens Healthcare, Erlangen, Germany), based on heart deformation analysis (HDA) as described in previous studies[5,6]. Left ventricular global longitudinal strain (LVGLS) was measured from standard 4-chamber images. LV global radial strain (LVGRS) and LV global circumferential strain (LVGCS) were measured using three short-axis images (base, middle, and apex), shown in figure 1. Myocardial T1, T2, and ECV values were determined by drawing regions of interest (ROIs) in each segment on a dedicated workstation with an ROI measuring tool (Siemens Healthcare, Erlangen, Germany), shown in Figure 2. The Student’s t-test was used to compare two groups of normally distributed variables. Pearson’s or Spearman’s correlation coefficients were used to test the correlations between the mapping data, and myocardial strain, and basic CMR functional parameters.Results
The basic functional parameters of the HT patients (including LVEF, EDV, ESV, SV, and LVMI) were significantly different from volunteers (all p <0.05). HT patients had higher native T1, T2. and ECV values (Native T1: 1043 ± 54 vs 1015 ± 24, p=0.015; T2: 48 ± 3 vs 46 ± 2, p=0.001; ECV: 27 ± 5 vs 24 ± 2, p=0.016, respectively) than volunteers. Lower post-T1 (440 ± 25 vs 455 ± 21, p=0.048) were also seen. Left ventricular global strain (LVGLS), LV circumferential strain (LVGCS), and LV radial strain (LVGRS) in HT patients were different from volunteers (LVGLS: -11.0± 2.4 vs -15.1 ± 2.1, p< 0.001; LVGCS: -13.5 ± 2.3 vs -16.4 ± 2.1, p <0.001 and LVGRS: 31.2 ± 9.5 vs 36.8 ± 7.6, p=0.034). Additionally, the time-to-peak (TTP) of LVGLS, LVGCS, and LVGRS in HT patients were lower than those of volunteers (all p <0.05). No significant associations between LVEF and tissue parameters were seen; LVGCS and LVGRS were correlated with LVEF (r=-0.455, p=0.009 and r=0.549, p=0.001). Native T1 values were associated with LVGCS-TTP and LVGRS-TTP (r=0.508, p=0.003 and r=0.482, p=0.005), T2 was correlated with LVGCS-TTP (r=0.379, p=0.032). Post-T1 values were associated with LVGLS-TTP and LVGCS-TTP (r=0.390, p=0.033 and r=-0.407, p=0.021). ECV values were associated with LVGCS-TTP and LVGRS-TTP (r=0.442, p=0.011 and r=0.485, p=0.005).Discussion
In this study, the tissue parameters (T1, T2, and ECV) were elevated in HT patients, and could reflect myocardial edema and fibrosis[7,8]. Additionally, the cardiac function structural measurements (LVGLS, LVGCS, and LVGRS) in HT patients were different from those of volunteers, showing that myocardial strain measurements could be a sensitive tool to reflect abnormal myocardial movements. In this study, moderate correlations between CMR-myocardial strain and tissue parameters were seen. A possible reason for these results includes that myocardial edema and heart fibrosis could cause myocardial stiffness and abnormal movements. Additionally, LVGCS and LVGRS are related to LVEF derived from cardiac short-axis slices and myocardial short-axis movements.Conclusions
Myocardial tissue parameters, including T1, T2, and ECV maps and function parameters (LVGLS, LVGCS, LVGRS, and TTP) of HT patients differed from those of volunteers. Moreover, a moderate correlation between the tissue and functional parameters of these patients, indicating myocardial edema and fibrosis could cause abnormal myocardial movements.Acknowledgements
No acknowledgement found.References
[1] Badano LP, Miglioranza MH, Edvardsen T, et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. European Heart Journal-Cardiovascular Imaging, 2015, 16(9):919-948
[2] Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiological Reviews, 1999, 79(1):215-262
[3] Park S-J, Cho SW, Kim SM, et al. Assessment of Myocardial Fibrosis Using Multimodality Imaging in Severe Aortic Stenosis Comparison With Histologic Fibrosis. Jacc-Cardiovascular Imaging, 2019, 12(1):109-119
[4] Dolan RS, Rahsepar AA, Blaisdell J, et al. Cardiac Structure-Function MRI in Patients After Heart Transplantation. Journal of Magnetic Resonance Imaging, 2019, 49(3):678-687
[5] Guetter C, Xue H, Chefd'hotel C, et al. EFFICIENT SYMMETRIC AND INVERSE-CONSISTENT DEFORMABLE REGISTRATION THROUGH INTERLEAVED OPTIMIZATION. 2011 8th Ieee International Symposium on Biomedical Imaging: from Nano to Macro, 2011:590-593
[6] Liu H, Yang D, Wan K, et al. Distribution pattern of left-ventricular myocardial strain analyzed by a cine MRI based deformation registration algorithm in healthy Chinese volunteers. Scientific Reports, 2017, 7(
[7] Ide S, Riesenkampff E, Chiasson DA, et al. Histological validation of cardiovascular magnetic resonance T1 mapping markers of myocardial fibrosis in paediatric heart transplant recipients. Journal of Cardiovascular Magnetic Resonance, 2017, 19(
[8] Tahir E, Sinn M, Bohnen S, et al. Acute versus Chronic Myocardial Infarction: Diagnostic Accuracy of Quantitative Native T1 and T2 Mapping versus Assessment of Edema on Standard T2-weighted Cardiovascular MR Images for Differentiation. Radiology, 2017, 285(1):83-91
Figures