0990
Relaxivity Contrast Imaging (RCI) of Myocardial Microstructure in Secondary Cardiomyopathy
Sudarshan Ragunathan1, Ryan K Robison2, Erik G Ellsworth3, and C Chad Quarles1
1Barrow Neurological Institute, Phoenix, AZ, United States, 2Philips, Nashville, TN, United States, 3Phoenix Children's Hospital, Phoenix, AZ, United States
1Barrow Neurological Institute, Phoenix, AZ, United States, 2Philips, Nashville, TN, United States, 3Phoenix Children's Hospital, Phoenix, AZ, United States
Synopsis
Clinical cardiac MRI protocols can be insensitive to secondary cardiomyopathy induced myocardium thickening and altered cardiac function. In this work, we demonstrate the feasibility of acquiring cardiac relaxivity contrast imaging (RCI) data, and specifically the RCI-parameter termed transverse relaxivity at tracer equilibrium (TRATE). TRATE has previously been shown to detect changes in cellular microstructure. RCI acquisition comprises of 1) pre-/post-contrast T1 maps using a MOLLI protocol, and 2) pre-/post-contrast T2* maps using a multi-gradient echo protocol. Robust changes in post contrast R2*, and the subsequent TRATE values, support the potential role of using RCI to study myocardial microstructure changes.
INTRODUCTION
Cardiac magnetic resonance imaging (MRI) is commonly used to monitor treatment and progression of cardiomyopathies which can arise spontaneously or as a consequence of a secondary condition (e.g., congenital heart diseases like Tetralogy of Fallot, genetic disorders like Duchenne Muscular Dystrophy (DMD), hypertension, coronary artery disease)1,2. In both situations, changes to cardiomyocyte size and density can lead to abnormalities in the myocardial structure and function. Current clinical MR protocols focus on identifying morphologic and functional deficits and are relatively insensitive to changes to the underlying myocardial microstructure. Relaxivity contrast imaging (RCI) has been shown to detect tissue cytoarchitectural changes in brain tumor3 and muscle 4 by quantifying the transverse relaxivity at tracer equilibrium (TRATE). In this work we demonstrate the feasibility of RCI to detect myocardial microstructural changes in a small pediatric population.METHODS
As part of an IRB approved retrospective study, data was evaluated from cardiac MRI studies of 12 pediatric patients (age = 10.8 ± 4.9) acquired on a Philips 1.5T scanner. Each study involved injection of a gadolinium-based contrast agent (Gd-DOTA). Relaxivity contrast imaging requires the quantification of T1 and T2* changes pre- and post-injection of contrast agent. T1 mapping was performed using a MOLLI protocol [FOV = 300x300 mm2, voxel size = 2x2 mm2, slice thickness = 10 mm, SENSE factor = 2, a single 12 second breath-hold], and T2* changes were estimated using a multi- echo gradient echo (MGE) protocol [FOV = 310x310 mm2, voxel size = 1.9x1.9 mm2, slice thickness = 8 mm, SENSE factor = 1.5, 15 echoes, T2 prep pulse with default delay pre-contrast and 200 ms delay post-contrast, 15 second scan time, cardiac triggered, respiratory gated]. The MOLLI sequences were tuned to longer T1 values pre-contrast and shorter T1 values post-contrast. Both sequences were 2D acquisitions with a short axis view.The RCI parameter TRATE was computed as the ratio of $$$\frac{\Delta R_2^*}{C_t}$$$ at contrast agent (CA) equilibrium where, Ct is the CA (Gd-DOTA) concentration, computed as $$$\frac{\Delta R_1}{r_1}$$$. TRATE was estimated in two regions of interest (ROIs): the myocardium and the interventricular septum. The ROIs were manually selected for each patient. The data analysis was performed in MATLAB (MathWorks Inc).
RESULTS
The R2* distributions pre- and post- contrast injection in the ROIs are shown in Fig 1. Across all patients, ∆R2* [mean ± std] in the myocardium was estimated at 11.1 ± 8.5 s-1 and in the interventricular septum was estimated at 9.2 ± 7.0 s-1. Across all patients, TRATE [mean ± std] in the myocardium was estimated at 18.3 ± 8.2 mM-1s-1 and in the interventricular septum was estimated at 15.7 ± 5.4 mM-1s-1. Figure 2 highlights the spatial distribution of ∆R2* and TRATE in a representative patient in both ROIs. Localized regions with elevated TRATE within the myocardial wall but outside the septum were not uncommon as can be observed in Fig. 2. TRATE distributions across patients in both ROIs are presented as boxplots in Fig. 3. The median TRATE in the myocardium was 17.0 mM-1s-1, while the median in the septum was 14.6 mM-1s-1. TRATE in the entire myocardium was found to be significantly higher than TRATE in the septum.DISCUSSION
As a proof of concept, we have estimated TRATE using single time point acquisitions pre- and post-contrast injection. The success of applying RCI to study tissue microstructure in brain tumors and in muscle degeneration in ALS patients was the motivating factor behind extending its application to cardiac MR to study myocardial microstructure changes. Secondary cardiomyopathies exhibit microstructural abnormalities such as myocardial thickening and changes to cardiac function which cannot be characterized by current clinical imaging protocols that focus on anatomical and functional imaging. RCI is sensitive to the local field perturbations generated from the distribution of CA around cardiomyocytes and can potentially provide quantitative information unique to the cardiomyocyte size and density. In this work we observed significant T2*/R2* changes which were reflected by the TRATE values. Importantly, the TRATE values were three to four times higher than the CA’s r2 relaxivity (~ 4 mM-1s-1), confirming the biophysical feasibility of cardiac RCI. The contrast agent concentration did not vary substantially, indicating that the TRATE values were primarily influenced by ∆R2*. To our knowledge, this is the first study presenting RCI data in the myocardium. Future work will also include characterizing TRATE in healthy individuals, additional patients and correlation of TRATE with clinical markers of cardiac health and outcomes.CONCLUSION
The clinical feasibility of RCI in studying secondary cardiomyopathies in pediatric population was demonstrated as a proof-of-concept study. Further work is required to establish RCI as a potential imaging biomarker of myocardial microstructural abnormalities.Acknowledgements
This work was supported in part by Philips Healthcare.References
- Kolentinis M, Le M, Nagel E, Puntmann VO. Contemporary cardiac MRI in chronic coronary artery disease. European Cardiology Review . 2020;15. doi:10.15420/ecr.2019.172.
- Verhaert D, Richards K, Rafael-Fortney JA, Raman S v. Cardiac involvement in patients with muscular dystrophies magnetic resonance imaging phenotype and genotypic considerations. Circulation: Cardiovascular Imaging. 2011;4(1):67-76. doi:10.1161/CIRCIMAGING.110.9607403.
- Semmineh NB, Xu J, Skinner JT, et al. Assessing tumor cytoarchitecture using multiecho DSC-MRI derived measures of the transverse relaxivity at tracer equilibrium (TRATE). Magnetic Resonance in Medicine. 2015;74(3):772-784. doi:10.1002/mrm.254354.
- Ragunathan S, Bell L, Stokes A, et al. Evaluating Longitudinal Muscle Degeneration in ALS Patients using MR Cytography. In: Proceedings of International Society for Magnetic Resonance in Medicine . ; 2020.
Figures
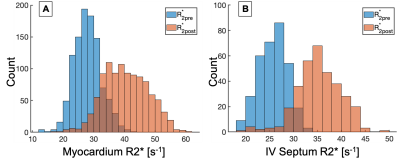
Figure 1: R2* distributions in the entire myocardium (A) and interventricular septum (B) are shown for pre- and post-contrast acquisitions. In both ROIs, post contrast R2* is markedly greater than the pre contrast R2*
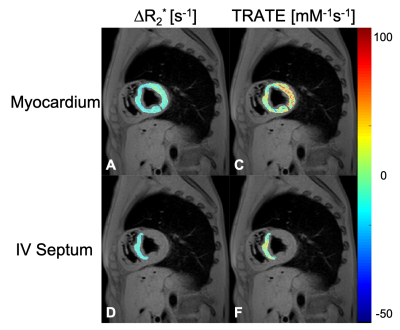
Figure 2: ∆R2* [s-1] and TRATE [mM-1.s-1] maps are shown for the myocardium (A,C) and the interventricular septum( D,F) regions of interest overlaid on a gradient echo anatomical image. The ROIs were drawn manually. These maps were generated from a representative dataset.
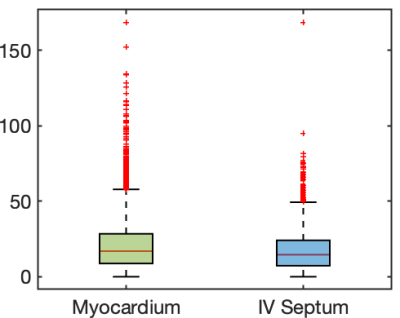
Figure 3: Box plots of TRATE values across all patients in the entire myocardium and interventricular septum. Data from the ROIs of all patients were used to generate the box plots.