0989
Myocardial fibrosis in preserved ejection fraction hypertrophic cardiomyopathy: predictive values of non-invasive markers1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2MR Collaboration, Siemens Healthineers Ltd, ShangHai, China
Synopsis
This study aimed to determine the predictive value of non-invasive markers in patients with hypertrophic cardiomyopathy with preserved ejection fraction. The results demonstrated that an elevated level of N-terminal pro b-type natriuretic peptide (Nt-proBNP) was an independent predictor for the presence of late gadolinium enhancement (LGE), and combined with maximum wall thickness (MWT), had good diagnostic performance for the detection of LGE. Furthermore, decreased left ventricular global circumferential strain (GCS) was independently correlated with the LGE%, indicating its potential prognostic value for detecting myocardial fibrosis.
Background
Myocardial fibrosis, as assessed by late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR), is associated with cardiovascular outcomes in hypertrophic cardiomyopathy (HCM) patients1,2. Myocardial fibrosis in HCM patients has become an important clinical problem and might lead to arrhythmias, advanced clinical events, and even sudden cardiac death3,4. Notably, myocardial fibrosis may be reversible and has been suggested as a powerful therapeutic target and prognosticator5,6. Therefore, identifying the non-invasive biomarkers for the early detection and prediction of myocardial fibrosis could have a role in management and risk stratification in HCM. However, little is known about the utility of non-invasive markers for detecting LGE. The aim of this study was to explore the association between cardiac-specific biomarkers, CMR myocardial strain, left ventricular (LV) hypertrophy, and LGE in HCM patients with preserved ejection fraction (EF); these indices for LGE are further investigated to assess their predictive values.Materials and Methods
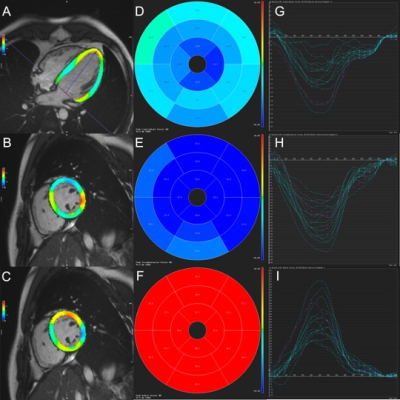
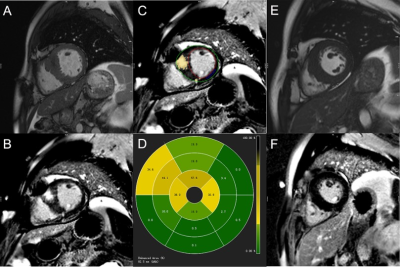
The study consisted of 86 HCM patients with preserved EF (mean age: 52.8 ± 11.9 years; 70 males) and 33 healthy volunteers (mean age: 47.9 ± 10.4 years; 25 males) who underwent contrast-enhanced CMR examinations. All CMR scans were acquired on a 1.5T system (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) with standard vector-electrocardiographic gating. LV function, end-diastolic maximum wall thickness (MWT), three-dimensional global peak systolic strains (Figure 1), and extent of LGE (% LGE) (Figure 2) were assessed. Baseline clinical and biochemical indices were recorded before the CMR examination. Multivariate linear regression analyses and receiver operating characteristic (ROC) curve analyses were used to assess the utility of each non-invasive markers for detecting LGE.Results
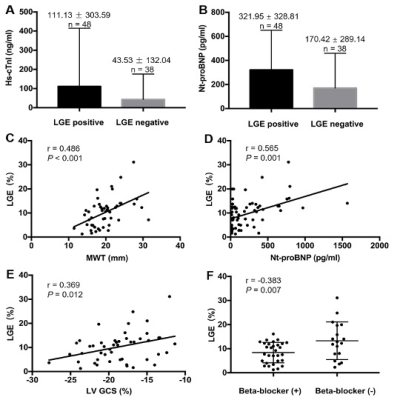
In total, 48 of 86 HCM patients showed LGE (mean LGE levels: 10.2 ± 6.2%). Compared with LGE-negative patients, serum high-sensitivity cardiac troponin I (hs-cTnI) and N-terminal pro b-type natriuretic peptide (Nt-proBNP) levels were elevated in LGE-positive patients (Figure 3: A, B). LGE-positive patients also had lower global longitudinal (GLS) and circumferential (GCS) strains than the LGE-negative group and healthy controls (p<0.05 for all). LGE% was independently associated with the Nt-proBNP levels (standardized β=0.627, p<0.001), GCS (standardized β=0.406, p=0.013), MWT (standardized β=0.481, p=0.001) and beta-blocker treatment (standardized β=-0.372, p=0.01) (Figure 3: C-F). In ROC curve analysis, the combined parameters of Nt-proBNP≥108 pg/mL and MWT≥17.3 mm showed good diagnostic performance for LGE, with a specificity of 81.3% and sensitivity of 70.0% (Figure 4).Discussion
This study showed that elevated levels of Nt-proBNP were significantly correlated with the extent of LGE and were an independent predictor of the presence of LGE. These correlations may be a result of myocardial fibrosis that could promote diastolic dysfunction and abnormal microcirculation, leading to ischemia and replacement scarring7-9. This suggests there is adequate pathophysiological background to consider investigating the potential of Nt-proBNP as a biomarker reflecting myocardial fibrosis. We also showed that GCS is not only independently associated with LGE%, but also useful for detecting myocardial fibrosis based on ROC curve analysis; therefore, GCS may have clinical utility for reflecting the presence of LGE. Additionally, Nt-proBNP ≥ 108 pg/mL and MWT ≥ 17.3 mm had excellent diagnostic performance for the detection of LGE on CMR. These data combining non-invasive clinical biomarkers and imaging technology for identifying myocardial fibrosis demonstrate potential clinical utility in early detection of patients with a poor prognosis.Conclusion
Serum Nt-proBNP is a potential biomarker associated with LGE%, and combined with MWT is useful for identifying myocardial fibrosis in HCM patients with preserved EF. LV GCS may also be a more sensitive indicator for reflecting the presence of myocardial fibrosis than GLS.Summary of Main Findings
Non-invasive markers, such as serum Nt-proBNP, MWT and GCS, were shown to be associated with the presence of LGE in HCM patients with preserved ejection fraction.Acknowledgements
We are very grateful for all the participants who have been enrolled in the study and all colleagues for helping us during the current study. This work was supported by Hubei Province Key Laboratory of Molecular Imaging (02.03.2018-90) and Union Hospital, Huazhong University of Science and Technology (02.03.2019-101).References
1. Mentias A, Raeisi-Giglou P, Smedira NG, et al. Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Preserved Systolic Function. J Am Coll Cardiol. 2018; 72(8):857-870.
2. Freitas P, Ferreira AM, Arteaga-Fernandez E, et al. The amount of late gadolinium enhancement outperforms current guideline-recommended criteria in the identification of patients with hypertrophic cardiomyopathy at risk of sudden cardiac death. J Cardiovasc Magn Reson. 2019; 21(1):50.
3. Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart. 2015; 101(17):1406-1411.
4. Maron MS, Chan RH, Kapur NK, et al. Effect of Spironolactone on Myocardial Fibrosis and Other Clinical Variables in Patients with Hypertrophic Cardiomyopathy. Am J Med. 2018; 131(7):837-841.
5. Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002; 105(21):2512-2517.
6. Izawa H, Murohara T, Nagata K, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005; 112(19):2940-2945.
7. Hasegawa K, Fujiwara H, Doyama K, et al. Ventricular expression of brain natriuretic peptide in hypertrophic cardiomyopathy. Circulation. 1993; 88(2):372-380.
8. Tesic M, Seferovic J, Trifunovic D, et al. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J Cardiol. 2017; 70(4):323-328.
9. Petersen SE, Jerosch-Herold M, Hudsmith LE, et al. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007; 115(18):2418-2425.
Figures