0986
Parametric mapping to assess cardiotoxicity in breast cancer patients receiving concurrent anthracyclines and trastuzumab1Radiology, Christian Medical College Vellore, Vellore, India, 2Radiology, Christian Medical College, Vellore, Vellore, India
Synopsis
Novel cardiac MRI mapping techniques such as T1, T2 and ECV parametric mapping have been proven to detect early cardiotoxicity among patients taking chemotherapeutic drugs. We aimed to determine whether these parameters could predict the development of early onset cardiac dysfunction in HER 2 positive breast cancer patients who were on anthracyclines and trastuzumab. We demonstrated statistically and clinically significant drop in left ventricular ejection fraction (LVEF) and right ventricular ejection fraction (RVEF) at four months post chemotherapy. The change in the mapping values were not statistically significant, however there was a trend towards an increase in the ECV.
Introduction
World-wide there has been an improvement in breast cancer survival, due to novel chemotherapeutic drugs; the drawback to this has been an increasing incidence of the adverse effects of cancer chemotherapeutic drugs, the most lethal being cancer therapy related cardiac dysfunction (CTRCD)1. There is an urgent need to validate modalities and algorithms for the early detection of cancer therapy induced cardiotoxicity. Current definitions of cardiotoxicity refer primarily to a drop in left ventricular ejection fraction (LVEF),2,3 however a normal LVEF does not exclude sub-clinical myocardial dysfunction.4,5 In women with HER 2 positive breast cancer, neoadjuvant chemotherapy with anthracycline and trastuzumab has been advocated. This regimen has been found to be effective in down staging the tumour and regional lymph nodes rendering the tumour operable. However, these are known to cause myocardial fibrosis and subsequent left ventricular dysfunction.6,7,8 In a randomized controlled phase III trial, cardiotoxicity in the form of significant LVEF decline was found at week 12 in one (0·8%) of 130 patients who received sequential treatment with anthracyclines and trastuzumab, and four (2·9%) of 137 patients who received concurrent treatment with anthracyclines and trastuzumab.9 This study aims to assess the early cardiotoxic changes that take place in the myocardial tissue using sequences of cardiac magnetic resonance imaging which include novel parametric mapping techniques such as T1, T2 mapping and ECV measurements which are useful for quantifying myocardial edema and fibrosis10. This was done to ascertain whether these methods of surveillance are superior to routine echocardiography in identifying early cardiotoxicity.Methods
We recruited HER 2 positive breast cancer patients who were on a concurrent regimen of anthracycline and trastuzumab. Based on previous studies, the sample size calculated was 18 cases11. Cardiac MRI scans were performed at 1.5-T Siemens MAGNETOM Avanto-fit MR machine, which included parametric mapping sequences as native T1, T2 and ECV provided as Work-in-progress solution by Siemens Healthineers, Germany. Left ventricular function and the parametric mapping/relaxometry values were contoured and obtained using standard post-processing software (Siemens syngo workstation). Concurrently an echocardiogram with global longitudinal LV strain (GLS) measurement was done. Echocardiogram and CMR were done before initiation of chemotherapy and for a total of two follow-ups, at 2 months and 4 months. Clinical and echo follow up was also done at 6-8 months. Cardiac biomarkers such as Troponin T and packed cell volume were also analyzed at each visit.Results:
- The majority of the patients enrolled in this study had stage IIIB of breast cancer, with a mean age of 45 years, and none of them had prior cardiac co-morbidities.
- There was a mild decrease in the collective CMR LVEF (60.9 ± 5.2 to 56.3 ± 6.5) and right ventricular EF (58.9 ± 8.3 to 51.7 ± 6.5) from baseline to second follow up, however, this was not a clinically significant decline.
- One patient demonstrated a clinically significant reduction in LV systolic function (LVEF) on cardiac MRI as well as echo-cardiography with a concomitant clinically significant reduction in CMR RV function( RVEF); however, she was asymptomatic and her parametric mapping values were normal and her subsequent follow up showed normal systolic function.
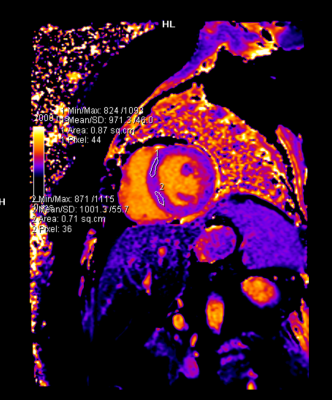
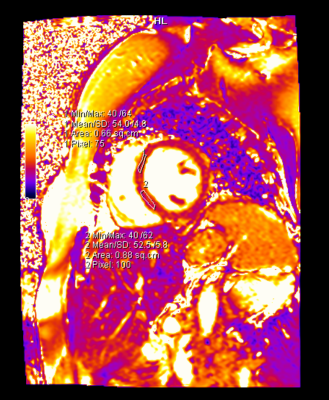
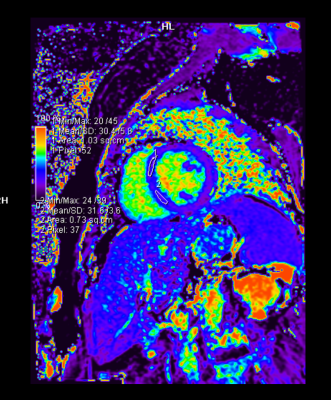
- The parametric mapping values (T1, T2, and ECV) did not change significantly. At the end of four months, one patient showed a mildly elevated T2 (58ms) and borderline elevated ECV (32.4%), and another patient demonstrated a borderline elevated ECV (32%) with a mildly reduced GLS value (-16%). These patients had normal LV and RV systolic function. Longer follow-up is awaited in these patients.
- At the 6 months follow-up, one patient was detected to have cardio-toxicity in the form of LV systolic dysfunction on echo (LVEF <50%) while she was on adjuvant chemotherapy with trastuzumab. During the initial 4 months, she had shown a borderline elevated ECV (32%), while the rest of her mapping values were normal. She remained asymptomatic, her trastuzumab therapy was interrupted for a short duration and she was initiated on cardioprotective medication.
Discussion
Our study demonstrated that both cardiac MRI and echocardiography are invaluable in detecting cardiotoxicity in the form of LVEF decline. Sixty percent (11 out of 18) of these breast cancer patients developed significant LVEF decline seen on either echocardiography or cardiac MRI, and the change in GLS was significant in 3 of these patients. In terms of detecting early cardiotoxicity, the native T1, T2 and ECV values, which were compared to our institutional standardized control values, showed no significant change, however there was a trend towards the increase in the ECV. We also confirmed that there was no significant difference between the global value and the septal mapping values.Conclusions
Similar to other studies in the literature, our study also shows that short term follows up may not optimally reflect the changes occurring in the myocardium due to chemotherapeutic drug toxicity. Anecdotal cases did show changes in some of the parameters. Larger sample size with a long-term follow-up is required to obtain a better picture of this disease entity.Acknowledgements
Dr. Binita Riya Chacko, Department of Radiology, CMC Vellore
Dr. Elizabeth Joseph, Department of Radiology, CMC Vellore
Dr. Leena RV, Department of Radiology, CMC Vellore
Dr. Aparna I, Department of Radiology, CMC Vellore
Dr. Jesu Kripa, Department of Cardiology, CMC Vellore
Dr. Ashish Singh, Department of Medical Oncology, CMC Vellore
The authors would also like to acknowledge the research and collaboration team from Siemens Healthineers support, India for their scientific and technical support
References
1. Jl P, T B, C D, D D, Td D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study [Internet]. Vol. 13, Breast cancer research : BCR. Breast Cancer Res; 2011 [cited 2020 Dec 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/21689398/
2. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). European Heart Journal. 2016 Sep 21;37(36):2768–801.
3. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014 Sep;27(9):911–39.
4. Čelutkienė J, Plymen CM, Flachskampf FA, de Boer RA, Grapsa J, Manka R, et al. Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018 Dec;20(12):1615–33.
5. K K, P O, Th M. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction [Internet]. Vol. 100, Heart (British Cardiac Society). Heart; 2014 [cited 2020 Dec 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/24860005/
6. Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009 Jun 16;53(24):2231–47.
7. Bn B, Jb C, Ck L, Mc F. Pathology of late-onset anthracycline cardiomyopathy [Internet]. Vol. 19, Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. Cardiovasc Pathol; 2010 [cited 2020 Dec 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/19747852/
8. Tm S, M P, Dj van V, M M, J B, C C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial [Internet]. Vol. 25, Journal of clinical oncology : official journal of the American Society of Clinical Oncology. J Clin Oncol; 2007 [cited 2020 Dec 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/17646669/
9. Buzdar AU, Suman VJ, Meric-Bernstam F, Leitch AM, Ellis MJ, Boughey JC, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. The Lancet Oncology. 2013 Dec;14(13):1317–25.
10. Kim PK, Hong YJ, Im DJ, Suh YJ, Park CH, Kim JY, et al. Myocardial T1 and T2 Mapping: Techniques and Clinical Applications. Korean Journal of Radiology. 2017;18(1):113.
11. Muehlberg F, Funk S, Zange L, von Knobelsdorff-Brenkenhoff F, Blaszczyk E, Schulz A, et al. Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy: Native T1 time can predict anthracycline-induced cardiomyopathy. ESC Heart Failure. 2018 Aug;5(4):620–9.
12. Meléndez GC, Jordan JH, D’Agostino RB, Vasu S, Hamilton CA, Hundley WG. Progressive 3-Month Increase in LV Myocardial ECV After Anthracycline-Based Chemotherapy. JACC: Cardiovascular Imaging. 2017 Jun;10(6):708–9.
13. Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013 Mar 1;111(5):717–22.
Figures