0983
Simultaneous dark blood late gadolinium enhancement and MR angiography for atrial scar detection1Center for Biomedical Imaging Research (CBIR), Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China, 3Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States, 4Department of Medical Engineering, Xinqiao Hosptial, Army Medical University, Chongqing, China, 5National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Cardiovascular magnetic resonance can provide functional and structural assessment of the left atrium and pulmonary veins, providing highly relevant pre- and post-procedural information in the treatment of atrial fibrillation (AF). In this study, an independent navigator-gated simultaneous dark blood late gadolinium enhancement and MR angiography sequence was developed for atrial scar detection. In 4 post AF ablation subjects, comparison with conventional phase sensitive inversion recovery images indicated that both scar-to-blood and blood-to-myocardium contrast were improved. Excellent correlation was observed between the scar measured by the proposed sequence and the low voltage areas observed with electroanatomical mapping.
INTRODUCTION
Radiofrequency ablation (RFA) was widely used for controlling Atrial fibrillation (AF), which is the most common clinically significant cardiac arrhythmia (1). Detailed visualization of atrial fibrosis and ablation scar in conjunction with surrounding anatomy can be acquired by combining multiple MRI sequences, which can improve the pre-procedural decision making, and post-procedural prognosis (2,3). The addition of magnetization preparation to late gadolinium enhancement (LGE) produces dark blood with an optimized contrast between atrial scar, myocardium and blood, and results in better delineation of the atrial wall and scar (4-6). Additionally, MR angiography (MRA) can provide structural imaging of left atrium (LA) and pulmonary vein, which has been used to detect the possible pulmonary vein stenosis after RFA (7), and helped the segmentation of LA (8). In this study, we aim to develop an independent navigator-gated simultaneous Dark Blood Phase Sensitive Inversion Recovery and MRA sequence (iDB-PSIR) for improved atrial imaging and scar detection. Co-registered multi-contrast high resolution images are acquired within single scan.METHODS
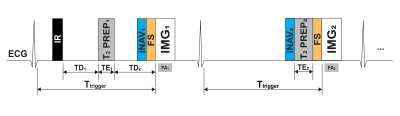
Pulse sequence: The proposed free breathing iDB-PSIR sequence, based on previous work (5), is illustrated in Figure 1. Two electrocardiogram (ECG) triggered 3D volumes are interleaved and acquired using multi-shot spoiled gradient echo. The magnetization sampled in the first volume (IMG1) is doubly prepared: inversion recovery (IR) and T2-preparation (T2PREP1) with echo time (TE1). The delay times between IR and T2PREP1 (TD1) and between T2PREP1 and IMG1 (TD2) are calculated using Bloch equation simulations to achieve dark blood LGE(4,5). The second volume (IMG2) is T2-prepared (T2PREP2) with echo time (TE2) to get bright blood MRA images, and also functions as the reference image for phase sensitive reconstruction. A lower flip angle (FA2) was used for IMG2 (9) compared with IMG1 (FA1). Before data acquisition, fat suppression (FS) is applied, and independent respiratory navigator (iNAV1, iNAV2) are performed for motion-compensation with high scan efficiency(10).In vivo experiments: Four AF patients (3 males, 56±13 years) 1-3 months post RFA were recruited, as approved by the local institutional review board. Written informed consent was obtained from all subjects. Imaging was performed on 3 T MR scanner (Ingenia CX, Philips Healthcare, Best, Netherlands). Both independent navigator-gated 3D PSIR and the proposed 3D iDB-PSIR sequences were acquired in axial orientation after contrast (Gd‐DTPA, Magnevist, 0.2mmol/kg) injection, at 10 and 20 mins in a random order respectively. A single slice Modified look‐locker inversion recovery (MOLLI) were performed before iDB-PSIR to measure the T1 values of remote left ventricular myocardium and blood pool, which were integrated into calculation of the subject-specific timing parameters TD1, TD2 (5). Typical imaging parameters were: FOV 280×280×100 mm3, TR/TE 5.2/2.6 ms, TE1 = 25ms TE2 = 35ms, voxel size 1.25×1.25×3 mm3 reconstructed into 0.73×0.73×1.5 mm3, SENSE acceleration factor 2, FA1= 18° FA2= 10°, acceptance window for navigator 5 mm. The ECG trigger delay (Ttrigger) was determined from cine of the atrium to image at its most quiescent period.
Regions of interest (ROIs) of atrial scar, blood and ventricular myocardium were manually defined on a representative slice. The image contrast between tissues were measured as the difference between mean signal intensity divided by the root of sum of square. A paired two-tailed Student's t-test was used for statistical analysis. The epi- and endocardial LA borders in iDB-PSIR images were manually delineated by two experienced cardiologists, and the scar quantification of whole LA was compared with that from electroanatomical mapping (EAM) recorded during the RFA procedure.
RESULTS
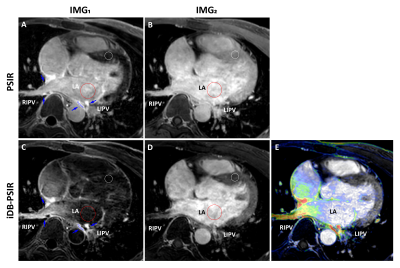
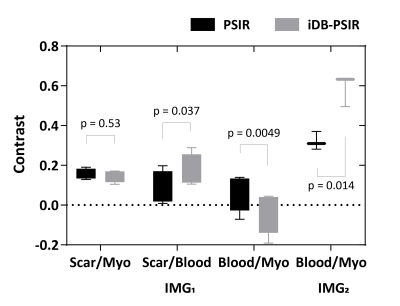
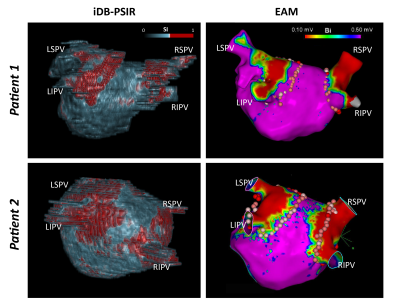
Imaging were successfully performed on all subjects. Two intrinsically co-registered high-resolution 3D volumes were acquired in 8.64±1.2 mins. Figure 2 shows representative slices of the two volumes acquired by 3D PSIR and the proposed iDB-PSIR. Compared with PSIR, iDB-PSIR could simultaneously acquire dark blood LGE and bright blood images. Figure 3 shows that IMG1 from iDB-PSIR had significantly higher scar-to-blood contrast (0.17±0.08 vs. 0.09±0.08, p=0.04) and significantly lower blood-to-myocardium contrast (-0.03±0.11 vs. 0.07±0.10, p<0.01) than PSIR, without significant difference in scar-to-myocardium contrast (0.15±0.03 vs. 0.16±0.03, p=0.53). IMG2 from iDB-PSIR had higher blood to myocardium contrast than PSIR (0.59±0.08 vs. 0.32±0.05, p=0.01). Figure 4 compares 3D renderings from iDB-PSIR images and EAM. The hyper-enhanced area in LGE is related to the low voltage area around the pulmonary veins in EAM(11).DISCUSSION
In this study, a simultaneous dark blood LGE and MRA sequence was developed. Co-registered high-resolution 3D volumes could be acquired in less than 10 min using independent respiratory navigators without increasing imaging time. Atrial scar was better delineated in IMG1 of iDB-PSIR, as scar to blood contrast was improved with dark-blood preparation. IMG2 had bright blood contrast, improving visualization of the structure of the LA and PVs, making segmentation of the LA possible and applicable to LGE images without the need of registration. The scar measured by iDB-PSIR has been qualitatively compared with the low voltage area in EAM and excellent correlation was found, though more quantitative comparation of the scar content is needed in further study.CONCLUSION
The proposed 3D iDB-PSIR sequence enables detection of post-ablation scar in left atrium by dark-blood LGE and structural information of the left atrium with a bright-blood MRA, in post-ablation patients, and all in a single scan.Acknowledgements
No acknowledgement found.References
1. Marrouche NF, Wilber D, Hindricks G, et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation. JAMA 2014;311:498
2. Akoum N, Wilber D, Hindricks G, et al. MRI Assessment of Ablation-Induced Scarring in Atrial Fibrillation: Analysis from the DECAAF Study. J. Cardiovasc. Electrophysiol. 2015;26:473–480
3. Peters DC, Wylie J V., Hauser TH, et al. Detection of Pulmonary Vein and Left Atrial Scar after Catheter Ablation with Three-dimensional Navigator-gated Delayed Enhancement MR Imaging: Initial Experience 1. Radiology 2007;243:690–695
4. Kellman P, Xue H, Olivieri LJ, et al. Dark blood late enhancement imaging. J. Cardiovasc. Magn. Reson. 2016;18:77
5. Si D, Wu Y, Yin J, et al. Detection of atrial scar using 3D high-resolution dark-blood phase sensitive inversion recovery imaging. In Proceedings of the 28th Annual Meeting of ISMRM, 2020.
6. Fahmy AS, Neisius U, Tsao CW, et al. Gray blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of myocardial scar. J. Cardiovasc. Magn. Reson. 2018;20:22
7. Hauser TH, Yeon SB, McClennen S, et al. Subclinical pulmonary vein narrowing after ablation for atrial fibrillation. Heart 2005;91:672–673
8. Malcolme-Lawes LC, Juli C, Karim R, et al. Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: A 2-center study. Hear. Rhythm 2013;10:1184–1191
9. Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn. Reson. Med. 2002;47:372–383
10. Lee S, Schär M, Zviman M, Sena-Weltin V, Harouni A, Kozerke S, McVeigh E, Halperin H, Herzka D. Free breathing independent respiratory navigator-gated imaging: concurrent PSIR and T2-weighted 3D imaging of the left ventricle. In Proceedings of the 19th Annual Meeting of ISMRM, Montreal, Canada, 2011. p 19.
11. Badger TJ, Daccarett M, Akoum NW, et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation; Lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ. Arrhythmia Electrophysiol. 2010;3:249–259
Figures