0982
Diffusion Tensor Imaging Reveals Myocardial Structure after Stem Cell-derived Cardiomyocyte Therapy in Myocardial Infarction1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2McEwen Centre for Regenerative Medicine, University Health Network, Toronto, ON, Canada, 3Schulich Heart Program, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 4Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada
Synopsis
Cardiac regenerative therapies using pluripotent stem cell-derived cardiomyocytes (PSC-CM, the graft) have shown promise in animal models of myocardial infarction. However, the mechanisms of structural integration of graft with host myocardium and the associated impact on function are less understood. 3D Diffusion Tensor Imaging (DTI) and contrast enhanced MRI were performed at 7T in ex vivo guinea pig hearts to quantify cardiac microstructure post PSC-CM transplantation. DTI parameters within the graft trended towards the healthy non-infarcted state, demonstrating a degree of anisotropic tissue repopulation within the infarct zone. MRI is a valuable marker for treatment efficacy in emerging regenerative therapies.
Background
Myocardial infarction (MI or a heart attack) remains the most common cause of heart failure (HF) worldwide1. HF has a mortality rate of ~50% over the first 5 years. Recent advances have improved survival during the early phase post-MI, but ~25% of survivors go on to develop HF2, a debilitating disease that significantly diminishes the quality of life for patients. The only clinically effective way to restore function in end-stage HF is through heart transplantation, which offers hope for only 10% of viable candidates3. Cardiac regenerative therapies using pluripotent stem cell-derived cardiomyocytes (PSC-CM) (the graft) have shown early promise in animal models of MI4, however, in vivo response, therapeutic efficacy and the associated mechanisms of repair remain less understood5. Diffusion Tensor Imaging (DTI) has demonstrated the ability to quantify myocardial structure in other cardiac regenerative strategies6. The objective of our study was to employ and optimize a 3D high-resolution DTI protocol to localize and characterize structural changes in the infarct and cellular graft regions after PSC-CM transplantation in an ex vivo guinea pig model of MI.Methods
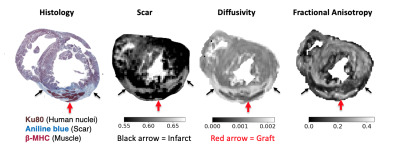
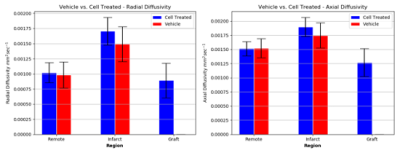
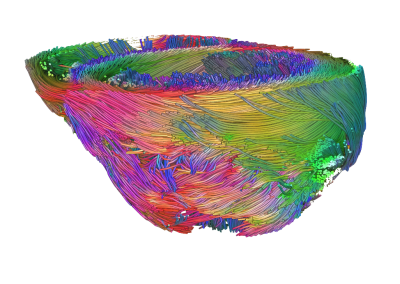
Guinea pigs were randomly selected into one of three groups, 1) PSC-CM treated (n=4), 2) vehicle treated (n=2), or 3) no treatment (healthy control, n=2). Guinea pig hearts of the treatment groups were cryoablated on the lateral wall of the left ventricle to induce infarction via thoracotomy. Infarct regions were injected either with PSC-CMs or vehicle on Week 2 post-MI. Hearts were harvested on Week 5 and perfusion fixed. Ex vivo MRI was performed on a 7T Bruker system. We utilized a stock 3D DTI sequence (resolution 0.3x0.3x0.3 mm, b-value 700 s/mm2, 30 diffusion directions, 5 b0 images) to quantify myocardial structure parameters such as fractional anisotropy (FA) and mean diffusivity (MD). DTI tractography was employed in order to qualitatively visualize myocardium and engrafted tissue. Contrast enhancement (CE, resolution = 0.2x0.2x1 mm) was used to distinguish infarcted from healthy tissue via accumulation of gadolinium-DTPA (0.3ml/kg) injected prior to sacrifice. Images were analyzed offline using open source software7. DTI parameters were measured in infarct, graft and remote myocardial territories.Results
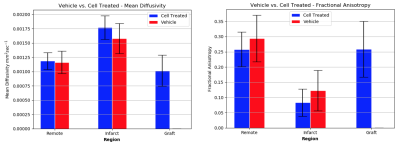
PSC-CM engraftment was in agreement between CE-MRI and histology. Results are presented as mean +/- standard deviation. Cell therapy subjects demonstrated a higher level of FA within the engraftment (0.26 +/- 0.0045) compared to the infarction (0.084 +/- 0.0026, p < 0.0001), and was similar to that of the healthy control (0.21 +/- 0.064, p < 0.0001). Likewise, MD of the graft region was not significantly different than that of the healthy control (1016 μm2s-1 +/- 148 vs. 1009 μm2s-1 +/- 207 respectively, p=0.41) and was improved compared to infarct (1770 μm2s-1 +/- 208, p< 0.0002).Discussion
To our knowledge, this is the first study to characterize tissue microstructure of the guinea pig myocardium following PSC-CM transplantation using DTI. FA and MD correlated to values reported in literature for murine myocardium8. The DTI measures within graft tissue were indicative of anisotropic structure, and showed greater myofibril organization compared to the infarcted territory. DTI parameters of graft tissue trended towards that of the healthy myocardium, indicating the grafted cardiomyocytes have a similar phenotype to healthy tissue. CE-MR images corresponded well to histological staining, demonstrating a non-invasive method of localizing the repopulated cardiomyocytes within the infarct territory.Conclusion
Our findings show that MRI serves to be a valuable non-invasive modality for graft localization and myocardial architecture characterization following cardiac regenerative therapy. Thus, DTI can help evaluate the degree of structural remodelling after MI and potentially how host-graft structural integration can impact functional recovery.Acknowledgements
We acknowledge funding support from Medicine by Design (MbD) Cycle 2, University of Toronto, CanadaReferences
1. Prevention of Recurrences of Myocardial Infarction and Stroke Study. (2013, December 18). World Health Organization.
2. Taylor, C. J., Ryan, R., Nichols, L., Gale, N., Hobbs, F. R., & Marshall, T. (2017). Survival following a diagnosis of heart failure in primary care. Family practice, 34(2), 161-168.
3. Taylor, D. O., Edwards, L. B., Boucek, M. M., Trulock, E. P., Aurora, P., Christie, J., Dobbels, F., Rahmel, A. O., Keck, B. M., & Hertz, M. I. (2007). Registry of the International Society for Heart and Lung Transplantation: Twenty-fourth official adult heart transplant report--2007. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation, 26(8), 769–781.
4. Romagnuolo, R., Masoudpour, H., Porta-Sánchez, A., Qiang, B., Barry, J., Laskary, A., ... Ghugre, NR., Keller, G., Laflamme, M. A. (2019). Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Reports, 12(5), 967–981.
5. Naumova, A. V., Modo, M., Moore, A., Murry, C. E., & Frank, J. A. (2014). Clinical imaging in regenerative medicine. Nature Biotechnology, 32(8), 804–818.
6. Nguyen, C. T., Dawkins, J., Bi, X., Marbán, E., & Li, D. (2018). Diffusion Tensor Cardiac Magnetic Resonance Reveals Exosomes From Cardiosphere-Derived Cells Preserve Myocardial Fiber Architecture After Myocardial Infarction. JACC: Basic to Translational Science, 3(1), 97–109.
7. Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, Nimmo-Smith I and Dipy Contributors (2014). DIPY, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, vol.8, no.8.
8. Angeli, S., Befera, N., Peyrat, JM. et al. A high-resolution cardiovascular magnetic resonance diffusion tensor map from ex-vivo C57BL/6 murine hearts. J Cardiovasc Magn Reson 16, 77 (2014).
Figures