0978
Assessment of cardiac aging in naked mole-rats and mice using ultrasound and MRI
Emine Can1, Megan Smith1, Rochelle Buffenstein1, and Johannes Riegler1
1Calico Life Sciences LLC, South San Francisco, CA, United States
1Calico Life Sciences LLC, South San Francisco, CA, United States
Synopsis
Naked mole-rats are extremely long-lived (>38 years) small (35-60g) rodents that show no increased risk of dying as they get older, implying that organ function is well-maintained throughout life. Using cardiac MRI, ultrasound and large cohorts of both mice and mole-rats we undertook a comprehensive comparative analysis of age-related changes in cardiovascular function over their lifespans. Unlike mice that show declines in most measurements of cardiac function and increased left ventricle wall thickness with age, we found naked mole-rats maintain cardiac function and functional cardiac reserve capacity well into their fourth decade of life.
Introduction
Both mice and humans share characteristic non-pathological features of cardiovascular aging, with peak cardiovascular function evident in early adulthood. Thereafter as these mammals age, changes in cardiac structure, vascular stiffness and functional decline become evident1. Not surprisingly, aging is the leading risk factor for cardiovascular diseases2, and it is critical to investigate mechanisms which could delay age-associated functional decline. The naked mole-rat (Heterocephalus glaber) may be an ideal organism with which to undertake such studies. At ages far beyond their expected maximum lifespan (~6 years), these small mammals show no increased risk of dying nor significant changes in physiological health3,4. Previous studies showed naked mole-rats maintain cardiac function up to 24 years of age5,6. However, with a lifespan of >38 years, changes at later ages are currently unknown. This study aims to elucidate age-related changes in cardiac function and functional reserve capacity in mice and naked mole-rats using high-resolution cardiac magnetic resonance imaging.Materials and Methods
Animals: C57BL/6J mice (0.3-to-2.5 years of age, ~31 g, 64 females, 73 males) were anesthetized with isoflurane (1.5 %) and body temperature was maintained at 37±0.4oC inside the MRI bore. Naked mole-rats (2-to-34 years of age, ~48 g, 48 females , 72 males) were anesthetized with isoflurane (2.5-3.5%) and their body temperature was maintained at 34±0.5oC during ultrasound acquisitions.MRI acquisition: Mouse cardiac MR images were acquired using a preclinical 9.4T (BioSpec 94/20 USR) horizontal bore scanner (Bruker, Ettlingen, Germany) with a shielded gradient system (660 mT/m). An animal monitoring system (Small Animal Instruments, NY) was used for ECG, breathing gating and heating control. Data acquisition was performed with a 4-channel phased array receive-only surface coil (Bruker) centered in a decoupled 86 mm transmit/receive volume coil (Bruker). Long- and short-axis scout images were acquired to define the two- and four-chamber long-axis views. The cine long-axis views were used to define short-axis slice stacks. A prospectively double-gated (ECG and respiration) spoiled gradient echo sequence with flow compensation was used with the following parameters: FOV 25x25x0.8 mm3, Matrix 192x192, TE 1.8 ms, TR 4-6 ms, FA 16o, NSA 1. Twenty cine-frames were recorded to cover the cardiac cycle. A single short-axis slice was obtained in ~50 seconds, leading to a total scan time of 10 minutes covering the heart from base to apex (12 slices).
Ultrasound (US) imaging: Naked mole-rat cardiac function was assessed using ultrasound imaging with a linear transducer (MX550D) operating at 40 MHz connected to a Vevo 3100 ultrasound system (Visualsonics, Toronto, Canada).
Cardiac stress test: Dobutamine hydrochloride 1.5 mg/kg diluted in saline (200 mg dobutamine/ml) was infused through an intraperitoneal catheter (1 ml/min). After a baseline scan, a second imaging stack (dobutamine stress) was acquired following a wait period of ~6 minutes to achieve sufficient dobutamine uptake in the heart.
Data analysis: Anonymized MRI data sets were analyzed using a semi-automatic segmentation software, Segment (Medviso AB, Sweden) while ultrasound data was analyzed using VevoLab software (Visualsonics). To test for correlations between functional parameters and time, linear models with time, time2 and sex as explanatory variables were evaluated. The model with smallest number of parameters that fitted the data best was selected. Differences between groups were considered significant if p < 0.05. Statistical analysis was performed using R software version 3.5.1.
Results and Discussion
Cardiac function of both species was investigated under basal and stress conditions using echocardiography and MRI (Fig. 1A and 1B). In mice, end diastolic and end systolic volumes increased linearly with age indicating a continuous dilation of the heart (Fig. 2A and B), in contrast, these volumetric measurements remained constant in naked mole-rats (Fig. 2E and F). Mouse left ventricular ejection fraction declined linearly with age (Fig. 2C) while no significant age-dependent change was observed in naked mole-rats (Fig. 2G). Left ventricular wall thickness showed a small decrease with age in naked mole-rats while it increased linearly in mice (Fig. 2D and 2H). Accordingly, no age-associated cardiac hypertrophy was observed in naked mole-rats.Upon administration of the adrenergic agonist, dobutamine, left ventricular contractility and heart rate substantially increased in both species. Unlike mice where age influenced dobutamine-induced increases in heart rate (Fig. 3A), age did not affect dobutamine-induced changes in naked mole-rats heart rate (Fig. 3D). Mice showed a linear decline in their ability to increase stroke volumes in response to dobutamine (Fig. 3B) resulting in similar age-related decline in normalized cardiac output (Fig. 3C). In contrast, in naked mole-rats dobutamine-induced myocardial contractility and stroke volume increased slightly with age (Fig. 3E) without changes in normalized cardiac output across age (Fig. 3F). Collectively, these findings indicate that unlike mice naked mole-rats, maintain baseline cardiac function and cardiac reserve as they get older.
Conclusion
High resolution cardiac MRI enabled the quantification of age-associated decline in mouse cardiac function which was not observed in naked mole-rats. Naked mole-rats thus provide a proof-of concept that age-related declines in cardiac function can be avoided. Understanding mechanisms involved in preventing age-related decline in naked mole-rat cardiac function may lead to new therapeutic targets for cardiovascular disease. Further studies are currently ongoing to validate ultrasound-derived results with cardiac MRI of naked mole-rats.Acknowledgements
We would like to thank members of the Buffenstein lab for support with naked mole-rat handling and husbandry.References
- Dai DF, Wessells RJ, Bodmer R, Rabinovitch PS. Cardiac Aging. In: The Comparative Biology of Aging, edited by Wolf NS, editor. Seattle, WA: Springer, 2010, p. 259–286
- North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097–1108. doi:10.1161/CIRCRESAHA.111.246876.
- Buffenstein, R. & Craft, W. in The Extraordinary Biology of the Naked Mole-Rat (eds R Buffenstein, TJ Park, & MM Holmes) Ch. 8, Springer Nature 2020. (ISBN 978-3-030-65942-4).
- Buffenstein, R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B 178, 439-445, doi:10.1007/s00360-007-0237-5 (2008).
- Grimes KM, Reddy AK, Lindsey ML, Buffenstein R. And the beat goes on: Maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. Am J Physiol - Hear Circ Physiol 2014;307. doi:10.1152/ajpheart.00305.2014.
- Ruby JG, Smith M, Buffenstein R. Naked mole-rat mortality rates defy gompertzian laws by not increasing with age. Elife 2018;7. doi:10.7554/eLife.31157.
Figures
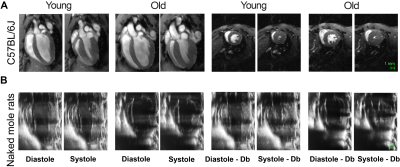
Fig. 1. (A) Representative magnetic resonance 4-chamber long axis images of a young (0.3 years) and old (2.5 years) mouse heart at end-diastole and end-systole and corresponding mid ventricular short axis images approximately 9 minutes after dobutamine infusion. (B) Representative ultrasound 2-chamber long axis images of a young (2.9 years) and old (27.4 years) naked mole-rat heart at end-diastole and end-systole and corresponding images after dobutamine infusion.
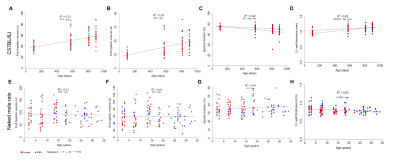
Fig. 2 Changes in baseline cardiac function in the aging heart. End diastolic and end systolic volumes increased linearly in mice (A, B) and remained constant in naked mole-rats (E, F). Left ventricular ejection fraction declined linearly in mouse (C) with no significant change in naked mole-rats (G). Left ventricular wall thickness increased linearly in mice (D) while it slightly decreased in naked mole-rats (H).
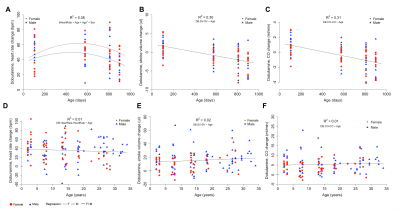
Fig. 3 Age-associated changes in cardiac function following dobutamine stimulation. The increase in the heart rate showed a quadratic response with a slight decrease in the oldest cohort in mice (A). In naked mole-rats, the change of heart rate was similar for all ages (D). The ability to increase stroke volumes and normalized cardiac output after dobutamine administration decreased linearly with age in mice (B, C). Dobutamine induced changes in stroke volume slightly increased in naked mole-rats (E) while increases in normalized cardiac output remained constant across age (F).