0976
Impaired CMR-derived Pulmonary Artery Global Longitudinal Strain is Related to Decompensated Hemodynamics in Pulmonary Arterial Hypertension
Shuang Leng1, Ru-San Tan1,2, Ping Chai3,4, Wen Ruan1, Ting Ting Low3,4, Lynette Teo4,5, Tee Joo Yeo3,4, Xiaodan Zhao1, John C. Allen2, Jonathan Yap1, Soo Teik Lim1,2, James W. Yip3,4, Ju Le Tan1,2, and Liang Zhong1,2
1National Heart Centre Singapore, Singapore, Singapore, 2Duke-NUS Medical School, Singapore, Singapore, 3National University Heart Centre, Singapore, Singapore, 4Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 5National University Hospital, Singapore, Singapore
1National Heart Centre Singapore, Singapore, Singapore, 2Duke-NUS Medical School, Singapore, Singapore, 3National University Heart Centre, Singapore, Singapore, 4Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 5National University Hospital, Singapore, Singapore
Synopsis
Pulmonary artery (PA) stiffness in pulmonary arterial hypertension (PAH) is strongly associated with clinical progression of the disease. This study aimed to introduce a novel parameter—PA global longitudinal strain (GLS)—for PA stiffness assessment using a semi-automated feature-tracking approach with standard cine cardiovascular magnetic resonance (CMR). Reduced PA GLS was significantly associated with increased pulmonary artery pressure and pulmonary vascular resistance in PAH. In addition, PA GLS was significantly impaired in PAH patients with hemodynamically decompensated right ventricular (RV) function compared to those with compensated RV function.
Background and Purpose
Pulmonary arterial hypertension (PAH) is a chronic and severe cardiopulmonary disorder that is characterized by a stiff pulmonary artery (PA) resulting in progressive elevation of pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR). PA stiffness is usually quantified as the ratio of change in luminal diameter of PA and change in pressure. However, the need for invasive pressure measurements limits its use for detection of early PA remodeling or for serial measurements. Pulse wave velocity (PWV) has been described as a noninvasive measure of arterial stiffness.1 However, it has not been adopted for clinical use because of difficulty in reliably measuring PWV and its dependence on hemodynamic conditions.2 In this study, we propose a novel parameter—PA global longitudinal strain (GLS)—to quantify PA stiffness in PAH based on a semi-automated strain measurement approach using standard cine cardiovascular magnetic resonance (CMR) images.Methods
Thirty-five PAH patients (48±12 years, 27 female) and 35 age- and sex-matched normal controls (47±14 years, 27 female) were enrolled (Table 1). PAH was diagnosed based on invasive hemodynamic measurements3: mean PAP ≥25 mmHg, pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, and PVR >3 Woods units. PAH patients were stratified into 2 groups by invasively measured mean right atrial pressures (RAP)4,5: PAH with hemodynamically compensated (PAH-C) and decompensated right ventricular (RV) function (PAH-D) with mean RAP <10 mmHg and ≥10 mmHg, respectively. All subjects underwent CMR scans on a 3T system (Ingenia, Philips Healthcare) or 1.5T system (MAGNETOM Aera, Siemens Healthineers). Standard end-expiratory breath-held cine images (steady-state free precession pulse sequence, retrospective electrocardiographic gating, typical temporal resolution 30 frames per cardiac cycle) were acquired in the PA bifurcation, RV outflow tract (RVOT), coronal RVOT, and RV 3-chamber views (Figure 1A) in addition to routine imaging planes. On these CMR views, the PA longitudinal strain parameters were measured using an in-house semi-automated algorithm6-9 that tracked the PA bifurcation and pulmonary valve in three-dimensional (3D) space: (1) landmarks on the PA bifurcation (red star) and pulmonary valve annulus (blue squares, yellow diamonds, red dots) were automatically tracked over the cardiac cycle (Figure 1A), (2) Two-dimensional (2D) spatial coordinates of the above points were mapped onto a 3D coordinate system, (3) the distance ($$$L$$$) between the PA bifurcation and pulmonary valve projected along the longitudinal direction was calculated, (4) instantaneous PA longitudinal strain at time point ($$$t$$$) relative to initial time point (time 0) at end-diastole was calculated using the formula for Lagrangian strain $$$\frac{(L(t)-L(0))\times100}{L(0)}$$$, and (5) PA GLS was defined as the peak absolute strain value (Figure 1B).Results
PA GLS assessment was successful in all subjects. Compared with controls, PAH patients had significantly lower PA GLS (10.0±3.5 vs. 19.4±4.8%, P<0.0001) (Table 1). There was a positive correlation between PA GLS and RV ejection fraction (EF) in the entire cohort (n=70, r=0.30, P=0.011). Among PAH patients, PA GLS was inversely correlated with mean RAP (r=-0.54, P=0.001), PVR (r=-0.43, P=0.018), mean PAP (r=-0.39, P=0.022), and PCWP (r=-0.52, P=0.005). PAH-D patients had significantly lower PA GLS than PAH-C (8.2±2.8 vs. 11.3±3.4%, P=0.007) (Table 1). On receiver operating characteristic (ROC) analysis, PA GLS (Area under the ROC Curve=0.77, Sensitivity=67%, Specificity=85%, Cut-off=8.6%) was superior to RVEF (AUC=0.64) for discriminating PAH-D from PAH-C.Conclusion
We have demonstrated the feasibility of measuring PA GLS, a novel cine CMR feature-tracking parameter for quantitation of PA stiffness. Impaired PA GLS was more prevalent among PAH patients compared with controls, was associated with elevated PAP and PVR, and discriminated well for RV decompensation in PAH. PA GLS may represent a useful non-invasive imaging index for characterization of PA stiffness as well as therapeutic monitoring in PAH.Acknowledgements
No acknowledgement found.References
- Friesen RM, Schäfer M, Ivy DD, et al. Proximal pulmonary vascular stiffness as a prognostic factor in children with pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging. 2019;20:209-217.
- Gupta A, Sharifov OF, Lloyd SG, et al. Novel noninvasive assessment of pulmonary arterial stiffness using velocity transfer function. J Am Heart Assoc. 2018;7:e009459.
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67-119.
- Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780-788.
- Simon MA, Rajagopalan N, Mathier MA, et al. Tissue Doppler imaging of right ventricular decompensation in pulmonary hypertension. Congest Heart Fail. 2009;15:271-276.
- Leng S, Ge H, He J, et al. Long-term prognostic value of cardiac MRI left atrial strain in ST-segment elevation myocardial infarction. Radiology. 2020;296:299-309.
- Leng S, Tan RS, Zhao XD, et al. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2018;20:71.
- Leng S, Dong Y, Wu Y, et al. Impaired CMR-derived rapid semi-automated right atrial longitudinal strain is associated with decompensated hemodynamics in pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2019;12:e008582.
- Leng S, Tan RS, Zhao XD, et al. Fast long-axis strain: a simple, automatic approach for assessing left ventricular longitudinal function with cine cardiovascular magnetic resonance. Eur Radiol. 2020;30:3672-83.
Figures
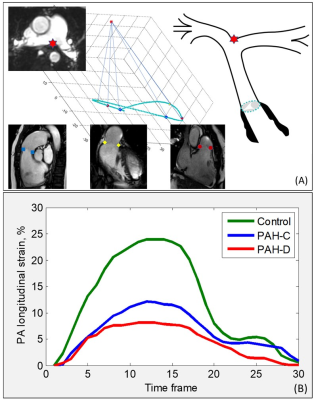
Figure 1. Pulmonary
artery (PA) global longitudinal strain (GLS) measurement using landmarks on the
PA
bifurcation (red star), right ventricular outflow tract (RVOT) (blue squares),
coronal RVOT (yellow diamonds), right ventricular (RV) 3-chamber (red dots)
views. (A) Distances between pulmonary valve and PA bifurcation were
automatically tracked in 3D space. (B) Derivation of PA GLS in one healthy subject,
one pulmonary arterial hypertension (PAH) patient with compensated RV function
(PAH-C), and one PAH patient with decompensated RV function (PAH-D).
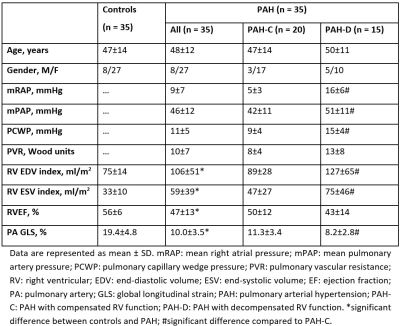
Table
1. Demographics,
clinical, hemodynamic, and pulmonary artery global longitudinal strain
measurements among subject groups.