0971
Cardiac mechanical performance of ALL survivors assessed by combined CMR and Incremental exercise test.1Mechanical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 2Kinesiology, Université de Montréal, Montreal, QC, Canada
Synopsis
Despite the late development of cardiac dysfunction in childhood acute lymphoblastic leukemia survivors, early detection of doxorubicin-related cardiotoxicity remains a challenge to better stratify these survivors who need a close follow-up. Cine-CMR acquisitions, combined to cardiopulmonary exercise testing and CircAdapt simulations showed that survivors’ exposition to doxorubicin, whatever the cumulative dose received, affected the cardiac efficiency and the ventricular-arterial coupling at rest and for light cardiac stress. Moreover, dexrazoxane treatments helped survivors to better adapt to high cardiac stress while presenting no differences at rest and for light cardiac stress.
Introduction
In childhood acute lymphoblastic leukemia (ALL) survivors, doxorubicin leads to dose-dependent cardiotoxicity, which is the most common cause of morbidity and mortality many years after the end of treatments [1]. CMR assessments of survivors who are undiagnosed of cardiac disease have shown increased ventricular volumes, increased left ventricular afterload, decreased left ventricular mass, decreased left ventricular contractility, decreased left ventricular wall thickness and dilated left ventricular end-diastolic dimension indicative of therapy-related injury [2-4]. Despite the late development of cardiac dysfunction in this population, early detection of doxorubicin-related cardiotoxicity remains a challenge to better stratify childhood cancer survivors who need a close follow-up. We hypothesize that the use of cardiopulmonary exercise testing (CPET) combined to CMR will unmask potential cardiac risks not observable in resting condition.Methods
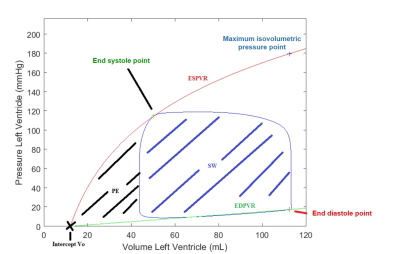
Sixty-six childhood ALL survivors (23±7 years) were included in this study approved by our IRB. Participants were classified into three prognostic risk group: standard risk (SR, n=14), high risk with and without cardio-protective agent dexrazoxane (HR, n=17 and HR+DEX, n=16). The CMR acquisitions were performed at rest on a Siemens Skyra 3T MR system using a 18-channel phased array body matrix coil and included an ECG-gated cine TruFISP sequence (14 slices in short axis and 5 slices in long axis, slice thickness 8mm, repetition time 34.6ms, effective echo time 1.2ms, flip angle 38°, iPAT factor 3, matrix 208x210 and in-plane pixel size 1.25x1.25 mm). The endocardial volume of the left ventricle was quantified from a semi-automatic segmentation (CIM v8.1, University of Auckland). Participants also underwent a maximal CPET (cycle ergometer, Oxycon Pro, Jaeger) with a standard incremental procedure where the load increased by 25W or 50W every 2min, depending on the height and sex of the participant. CPET was performed from rest to maximum stress (150-325W) and was coupled with a cardiac hemodynamic monitoring (PhysioFlow, Manatec Biomedical). The arterial pressure, cardiac output and heart rate measured at each stress step were used as input data to the CircAdapt model [5]. The CircAdapt mechanical properties of the left ventricle were computed from the reverse identification method using the hemodynamic data at rest. Then, for each stress step, the volume-pressure curves were analyzed (Figure 1). The cardiac work efficiency (CWE) was computed from the stroke work (SW) and the potential energy (PE) as CWE=SW/(SW+PE). The effective arterial elastance (Ea) was computed from the end systolic pressure (ESP) and the systolic ejection volume (VES) as Ea=ESP/VES. The ventricular elastance (Ees) was defined as the slope of the line connecting the intercept Vo to the maximum isovolumetric pressure point, and used to compute the ventricular-arterial coupling Ea/Ees.Results
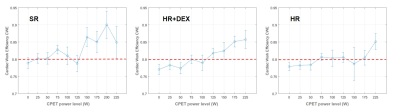
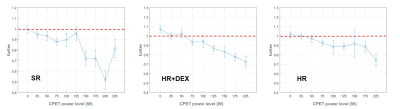
From 0W to 125W, CWE showed values around 80% for the three groups (Figure 2), which was lower than the 96% measured on healthy subjects [6]. From 125W to 225W, survivors in the SR group showed a higher cardiac efficiency than those in HR and HR+DEX groups. In the HR+DEX group, CWE increased linearly from 100W to 225W, while in the HR group, CWE only increased from 175W to 225W. The shape of the ventricular-arterial coupling curve was different between the three groups (Figure 3). For the SR group, Ea/Ees was stable from 0W to 125W and then decreased drastically from 125W to 200W. For the HR+DEX group, Ea/Ees decreased linearly from 0W to 225W. For the HR group, Ea/Ees decreased linearly from 0W to 100W, then stay stable from 100W to 175W and decreased from 175W to 225W. The decrease in the SR group was higher than the HR+DEX group, which was itself higher than the HR group. For all groups, almost all values were higher than 0.7, while healthy values are reported to be 0.6 [7].Discussion
Cardiac work efficiency and ventricular-arterial coupling showed doxorubicin effect on heart efficiency even at low dosage in all groups (HR, HR+DEX, and SR). At high level of CPET (175W-225W), the heart was well adapted in almost all groups. Howerver at average level of CPET (125W-150W), HR survivors hardly adapted to the effort. This is likely related to the significant cardiac sequelae of doxorubicin. During the CPET, exposition to dexrazoxane showed mostly his protective effect for the higher levels of stress. Early detection of cardiotoxicity is thus crucial and presents opportunity for personalized risk stratification and early therapeutic intervention before irreversible heart failure occurs [8]. Convergence issues were observed for the simulations of 18 of the 66 survivors at high stress levels (over 150W), which reduces the significance of the observed differences between groups. The definition of the ventricular elastance is still unclear in the literature, mostly when the ESPVR curve is not linear, which reduces the comparison to the literature.Conclusion
Survivors’ exposition to doxorubicin, whatever the cumulative dose received, affected the cardiac efficiency and the ventricular-arterial coupling at rest and for the first CPET steps. Moreover, dexrazoxane treatments helped survivors to better adapt to high cardiac stress while presenting no differences at rest and for light cardiac stress. Cardiac work efficiency and ventricular-arterial coupling tended towards healthy values while cardiac stress increased. It could be interesting to study these parameters after exercise induced cardiac remodeling.Acknowledgements
NSERC, FRQNT and Polytechnique Montreal for the financial support, researchers from the PETALE study for the opportunity to do this complementary analysis on the cancer survivors.References
1. Aissiou, M., et al., Imaging of early modification in cardiomyopathy: the doxorubicin-induced model. The international journal of cardiovascular imaging, 2013. 29(7): p. 1459-1476.
2. Armstrong, G.T., et al., Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol, 2012. 30(23): p. 2876-84.
3. Lipshultz, S.E., et al., Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med, 1991. 324(12): p. 808-15.
4. Lipshultz, S.E., et al., Chronic Progressive Cardiac Dysfunction Years After Doxorubicin Therapy for Childhood Acute Lymphoblastic Leukemia. Journal of Clinical Oncology, 2005. 23(12): p. 2629-2636.
5. Arts, T., et al., Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. Am J Physiol Heart Circ Physiol, 2005. 288(4): p. H1943-54.
6. El Mahdiui, M., et al., Global Left Ventricular Myocardial Work Efficiency in Healthy Individuals and Patients with Cardiovascular Disease. J Am Soc Echocardiogr, 2019. 32(9): p. 1120-1127.
7. Bastos, M.B., et al., Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J, 2020. 41(12): p. 1286-1297.
8. Burrage, M.K. and V.M. Ferreira, The use of cardiovascular magnetic resonance as an early non-invasive biomarker for cardiotoxicity in cardio-oncology. Cardiovasc Diagn Ther, 2020. 10(3): p. 610-624.
Figures