0967
Inter-observer and intra-observer agreement on the MRI-based left-ventricular contractility using compress-sensing accelerated cardiac MRI1Department of Diagnostic and Interventional Radiology, HKSH Eastern Medical Centre, Hong Kong, Hong Kong, 2HKSH Eastern Medical Centre, Hong Kong, Hong Kong
Synopsis
The reliability of the left-ventricular ejection function estimated from the compress-sensing accelerated cardiac MR scan was evaluated in the study. Excellent inter-observer and intra-observer agreement were observed based on the data from the compress-sensing cardiac MR scans, suggesting the usefulness of compress-sensing acceleration in improving the patient comfort and scan time reduction without degrading the reliability in the cardiac output assessment.
Purpose
The cancer treatment related cardiac dysfunction (CTRCD) has gained increasing concern since cardiaovascular disease is one of the leading cause of morbidity and mortality in cancer survivors. On one hand, the cancer survival rate improved with the advancement of the cancer therapies. On the other hand, several cancer treatments have been associated with various levels of acute and late-effect cadiovascular injury, which may lead to irreversible cardiac failure if left untreated. Early detection of CTRCD is, therefore, critical and may be done by monitoring the left-ventricular ejection function (LVEF) during the course of the cancer therapy. Although cardiac MRI (CMR) is the gold-standard for the cardiac function evaluation and is increasingly used to assess for cardiotoxicity, the scan is either done with multiple breath-hold, which reduced patient comfort, or respiratory trigger, which drastically prolonged the scan time. The recent developed compress-sensing accelerated cardiac MRI (CS-CMR) relieved patient discomfort by reducing the number of breath-holds during scans, while study on its reliability in LVEF estimation remains sparse. In this study, the inter-observer and intra-observer agreement on the LVEF estimation were evaluated using CS-CMR.Method
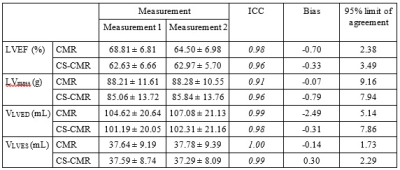
Cardiac MR scans of 12 female breast cancer patients (53.42 ± 7.82 years) were included in this study. The scan protocol during the adaption period of the new CS-CMR acquisition include both the conventional multiple breath-hold CMR protocol with GRAPPA acceleration (TR/TE = 37/1.3ms, FOV = 272x300mm, Flip angle = 80⁰, 25 cardiac phase, acquisition time = 86-120s, acceleration factor = 2, slice thickness = 8mm, matrix size = 256x256, voxel size =1.2x1.2x8.0mm3) and the 2 breath-hold CS-CMR protocol (TR/TE = 41/1.5ms, FOV = 267x300mm, Flip angle = 80⁰, 25 cardiac phase, acquisition time = 34-42s, acceleration factor = 9.5, slice thickness = 8mm, matrix size = 228x256, voxel size = 1.2x1.2x8.0mm3) performed on a 1.5T MR scanner. The left-ventricular boundary contours were manually segmented by two radiographers (>10 years of experience and >7 years of experience) for the inter-observer agreement assessment. For the intra-observer agreement assessment, the left-ventricular boundary contours were manually segmented separately twice with 1 month apart by the radiographer with >10 years of experience. For each contours, the left-ventricular mass (LVmass), left-ventricular end-diastolic volume (VLVED), left-ventricular end-systolic volume (VLVES) and LVEF were calculated using the manufacturer provided software package. The inter-observer and intra-observer agreement were assessed based on the Bland-Altman analysis and intra-class correlation (ICC).Results and Discussion
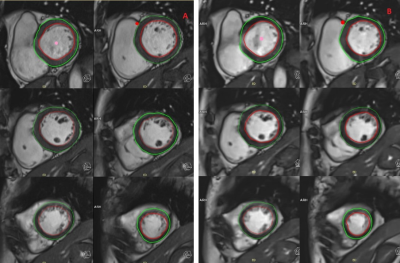
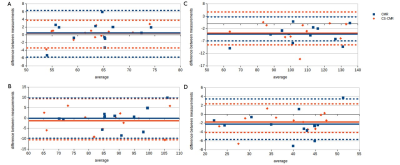
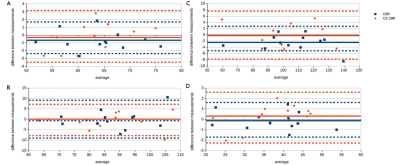
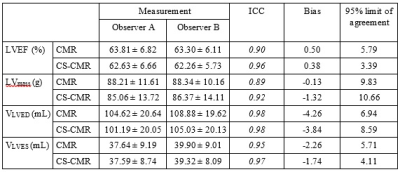
The location of the ROI delineated on CMR and CS-CMR scans performed by one of the radiographers was illustrated in Figure 1 with similar image quality between both techniques. As illustrated in Table 1, similar LVEF, LVmass, VLVED and VLVES were obtained by both observer using conventional CMR scan and CS-CMR scan for all parameters. Similarly, the intra-observer measurement for all parameters (LVEF, LVmass, VLVED and VLVES ) was shown in Table 2 for both CMR and CS-CMR scans. Excellent inter-observer agreement and intra-observer agreement were observed for both CMR scan and CS-CMR scans with ICC>0.9 for LVEF, LVmass, VLVED and VLVES (all p<0.05). It suggested that inter-observer and intra-observer agreement were high regardless of the scan technique (i.e. CMR or CS-CMR). Based on the Bland-Altman analysis, the bias was close to zero for both inter-observer (Figure 2) and intra-observer (Figure 3) using both CMR and CS-CMR scans.The inter-observer 95% limit of agreement of LVEF, LVmass, VLVED and VLVES were overall larger than or similar to the corresponding intra-observer 95% limit of agreements, suggesting the potential for further improvement in inter-observer agreement with better delineation consensus. Although larger intra-observer 95% limit of agreement was noted in CS-CMR than that of CMR for LVEF, VLVED and VLVES, the differences were, in fact, very small. More importantly, similar trend was not observed in the inter-observer 95% limit of agreement. It may indicate that compress-sensing acceleration does not degrade the inter-observer and intra-observer agreement of the left-ventricular output functions while drastically reducing the scan time by ~3-4 times (CS-CMR scan time = 38.7±2.5s; CMR scan time = 104.8±8.7s; p<0.0001).
This study may be limited by the small sample size, hence, a future study with larger sample size is warranted. Based on the results of this study, compress-sensing accelerated CMR provides a reliable and fast solution for cardiac function assessment, which is useful in the cardiac function evaluation and monitoring in cardiotoxicity.
Conclusion
The CS-CMR provided excellent inter-observer and intra-observer agreement in the left ventricular cardiac output assessment while relieving the patient discomfort with dramatic reduction in number of breath-holds and scan time. The results of this study may support the usefulness of compress-sensing acceleration in cardiac output function elevation and monitoring.Acknowledgements
No acknowledgement found.References
- Becker, M.; Frauenrath, T.; Hezel, F.; Krombach, G.A.; Kremer, U.; Koppers, B.; Butenweg, C.; Goemmel, A.; Utting, J.F.; Schulz-Menger, J.; Niendorf, T. Comparison of left ventricular function assessment using phonocardiogram- and electrocardiogram-triggered 2D SSFP CINE MR imaging at 1.5 T and 3.0 T. Eur. Radiol. 2010, 20, 1344–1355.
Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310.
Gharib MI, Burnett AK. Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. Eur J Heart Fail 2002;4:235–42.
Holloway, C.J.; Edwards, L.M.; Rider, O.J.; Fast, A.; Clarke, K.; Francis, J.M.; Myerson, S.G.; Neubauer, S. A comparison of visual and quantitative assessment of left ventricular ejection fraction by cardiac magnetic resonance. Int. J. Cardiovasc. Imag. 2011, 27, 563–569.
Kunz, R.P.; Oellig, F.; Krummenauer, F.; Oberholzer, K.; Romaneehsen, B.; Vomweg, T.W.; Horstick, G.; Hayes, C.; Thelen, M.; Kreitner, K.F. Assessment of left ventricular function by breath-hold cine MR imaging: Comparison of different steady-state free precession sequences. J. Magn. Reson. Imag. 2005, 21, 140–148.
Kido T, Kido T, Nakamura M, Watanabe K, Schmidt M, Forman C, et al. Compressed sensing real-time cine cardiovascular magnetic resonance: Accurate assessment of left ventricular function in a single-breath-hold. J Cardiovasc Magn Reson 2016; 18: 50.
Moon JC, Lorenz CH, Francis JM, Smith GC, Pennell DJ. Breath-hold FLASH and FISP cardiovascular MR imaging: Left ventricular volume differences and reproducibility. Radiology 2002; 223: 789 – 797.
Nassenstein, K.; Eberle, H.; Maderwald, S.;Jensen, C.J.; Heilmaier, C.; Schlosser, T.; Bruder, O. Single breath-hold magnetic resonance cine imaging for fast assessment of global and regional left ventricular function in clinical routine. Eur. Radiol. 2010, 20, 2341–2347.
Petitjean, C.; Dacher, J.N. A review of segmentation methods in short axis cardiac MR images. Med. Image Anal. 2011, 15, 169–184.
Vincenti G, Monney P, Chaptinel J, Rutz T, Coppo S, Zenge MO, et al. Compressed sensing single-breath-hold CMR for fast quantification of LV function, volumes, and mass. JACC Cardiovasc Imaging 2014; 7: 882 – 892.
Figures