0965
Evaluation of Isolated Left Ventricular Noncompaction Using Cardiac Magnetic Resonance Tissue Tracking in Strains1Department of Radiology, The Second Xiangya Hospital, Central South University, Changsha, China, 2MR Collaboration, Siemens Healthineers Ltd, Shanghai, China
Synopsis
We used tissue tracking cardiac magnetic resonance (TT-CMR) to quantitatively analyze the global, regional, and layer-specific strain of isolated left ventricular noncompaction (ILVNC). Combined with late gadolinium enhancement (LGE), we initially explored the effect of focal myocardial fibrosis on myocardial strain
Introduction
We used tissue tracking cardiac magnetic resonance (TT-CMR) to quantitatively analyze the global, regional, and layer-specific strain of isolated left ventricular noncompaction (ILVNC). Combined with late gadolinium enhancement (LGE), we initially explored the effect of focal myocardial fibrosis on myocardial strain.Methods
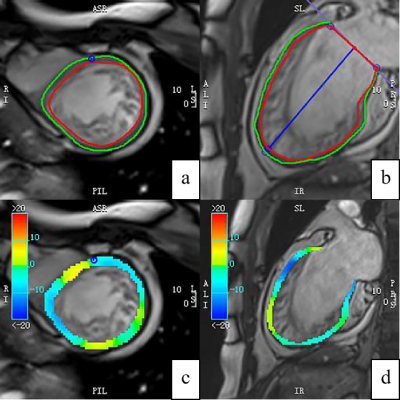
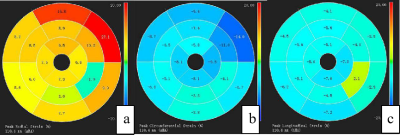
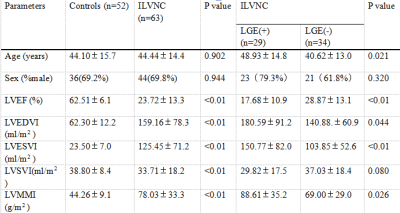
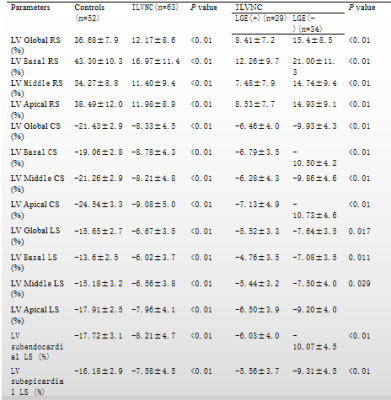
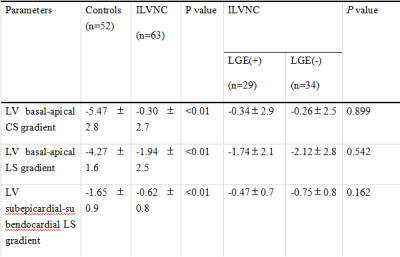
CMR was performed in 63 patients with ILVNC and 52 patients without ILVNC (i.e., the control group). The ILVNC group was divided into an LGE(+) group (29 patients) and an LGE(-) group (34 patients) according to the presence or absence of LGE. CVI42 software was used to measure global and regional (basal, middle, apical) radial strain (RS), circumferential strain (CS), longitudinal strain (LS), subendocardial LS, and subepicardial LS. The basal-apical strain gradient was defined as the apical mean strain minus the basal mean strain. We then compared differences between these strain parameters. The subendocardial-subepicardial LS gradient was defined as the maximum subendocardial LS minus the subepicardial LS.Results
Compared with the control group, the global and regional RS, CS, and LS, and the subendocardial and subepicardial LS of the ILVNC group were significantly diminished (P <0.01). Compared with the LGE(-) group, the global and regional RS, CS, LS, and the subendocardial and subepicardial LS of the LGE(+) group were significantly diminished (P <0.05). In the ILVNC group, the basal-apical CS and LS gradient, and the subendocardial-subepicardial LS gradient were significantly lower than those in the control group (P<0.01).Discussion
The purpose of this study was to explore the strain change patterns of ILVNC by quantitatively evaluating global, regional, and layer-specific myocardial strain. We also evaluated any correlation between LGE and strain in patients with ILVNC. In the early stages of embryo development, the myocardium gradually undergoes compaction from the subepicardium to the subendocardium,from the base to the apex.Failure of this process leads to abnormally large, disordered trabeculae and deep trabecular crypts, resulting in myocardial noncompaction 1. Therefore, the apical part is more severely affected than the basal part. In the current study, the apical segments of all patients with ILVNC were involved, and the compacted layer of the myocardium was significantly thinned.Analysis of regional strain found that in the control group, the apical CS and LS were significantly higher than that of the basal part; the reason for this is not clear but might be related to the geometry of the heart 2. The longitudinal and circumferential myocardial fibers integrate at the apex.During systole, the radius of curvature of the ventricular wall gradually decreases from the base to the apex, and the stress of the ventricular wall gradually decreases toward the apex. However, in the ILVNC group, there was no statistical difference between the basal and apical CS and LS. To evaluate the trend and extent of strain changes from the base to the apex, we defined the basal-apical strain gradient. The basal-apical CS and LS gradient in the ILVNC group was significantly lower than that in the control group; myocardial function decreased from the base to the apex in a non-homogeneous manner. This finding is consistent with those of Niemann et al. 3 and Haland et al. 4, who used tissue Doppler imaging and ultrasound speckle tracking to identify unique strain change patterns in ILVNC. The authors postulated that this was related to the compaction process of the embryonic myocardium from the base to the apex.CMR-TT can obtain subendocardial and subepicardial strain.We compared the subendocardial and subepicardial LS in the control group, observing that the subendocardial LS was higher. The presence of this gradient may be related to differences in the focal curvature between the subepicardial and subsubendocardial myocardium 5.However, in patients with ILVNC, no statistical difference was found between the subendocardial and subepicardial LS. Then we defined the subendocardial-subepicardial strain gradient. The current study results showed that the subendocardial-subepicardial LS gradient of the patients with ILVNC was significantly lower than that of the control group.That is, the degree of subendocardial myocardial involvement was relatively more severe. The subendocardial compacted myocardium was replaced by noncompacted myocardium, and the myocardium was grossly disordered. Therefore, in patients with ILVNC, subendocardial myocardial dysfunction is more pronounced than in the subepicardial myocardium.In the current study, the global and regional RS, CS, and LS of the LGE(+) group decreased compared to those of the LGE(-) group, demonstrating that myocardial fibrosis can cause limited myocardial movement in different directions. Further layer-specific studies of LS revealed that in patients with ILVNC with LGE (i.e., the LGE[+] group), subendocardial and subepicardial LS was also lower than in patients without LGE (i.e., the LGE[-] group), indicating that the myocardial layer-specific strain is a sensitive parameter. Quantitative analysis of subendocardium and subepicardium LS provides useful non-invasive information for the layer-specific localization of fibrosis 6.Conclusions
There were significant differences in myocardial strain between patients with and without ILVNC. ILVNC revealed a specific pattern in terms of strain change. The myocardial strain of the cardiac apex and endocardium was significantly lower than that of the cardiac base and epicardium, respectively. Myocardial strain reduction was more significant in ILVNC patients with focal myocardial fibrosis.Acknowledgements
This study was supported by the National Natural Science Foundation of China (NO.81701660).References
1. Captur G, Nihoyannopoulos P. Left ventricular non-compaction: genetic heterogeneity, diagnosis and clinical course. Int J Cardiol. 2010 Apr 15;140(2):145-53. doi: 10.1016/j.ijcard.2009.07.003. Epub 2009 Aug 6. PMID: 19664830.
2. Abou, R., Leung, M., Khidir, M., Wolterbeek, R., Schalij, M.J., Ajmone, M.N., Bax, J.J. & Delgado, V. Influence of aging on level and layer-specific left ventricular longitudinal strain in subjects without structural heart disease. Am J Cardiol. 120, 2065-2072 (2017).
3. Niemann, M., Liu, D., Hu, K., Cikes, M., Beer, M., Herrmann, S., Gaudron, P.D., Hillenbrand, H., Voelker, W., Ertl, G. & Weidemann, F. Echocardiographic quantification of regional deformation helps to distinguish isolated left ventricular non-compaction from dilated cardiomyopathy. Eur J Heart Fail. 14, 155-61 (2012).
4. Haland, T.F., Saberniak, J., Leren, I.S., Edvardsen, T. & Haugaa, K.H. Echocardiographic comparison between left ventricular non-compaction and hypertrophic cardiomyopathy. Int J Cardiol. 228, 900-905 (2017).
5. Alcidi, G.M., Esposito, R., Evola, V., Santoro, C., Lembo, M., Sorrentino, R., Lo, I.F., Borgia, F., Novo, G., Trimarco, B., Lancellotti, P. & Galderisi, M. Normal reference values of multilayer longitudinal strain according to age decades in a healthy population: A single-centre experience. Eur Heart J Cardiovasc Imaging. 19, 1390-1396 (2018).
6. Funabashi, N., Takaoka, H., Ozawa, K., Kamata, T., Uehara, M., Komuro, I. & Kobayashi, Y. Quantitative differentiation of LV myocardium with and without layer-specific fibrosis using MRI in hypertrophic cardiomyopathy and layer-specific strain TTE analysis. Int Heart J. 59, 523-530 (2018).
Figures