0964
Comprehensive Cardiac MRI Demonstrates Altered Myocardial Tissue and Function in Pediatric Heart Transplant Recipients1Pediatric Cardiology, Ann & Robert H Lurie Children's Hospital of Chicago, Chicago, IL, United States, 2Northwestern University, Chicago, IL, United States, 3Ann & Robert H Lurie Children's Hospital of Chicago, Chicago, IL, United States
Synopsis
Current gold standard for surveillance of pediatric heart transplant (PHT) complications is invasive and imperfect. CMR allows non-invasive evaluation of myocardial structure and function. A retrospective review of 26 PHT who underwent regadenoson stress perfusion CMR with tissue characterization and 18 age-matched controls was performed. Global T1 and ECV were higher in PHT; as were biventricular volumes and cardiac output. Myocardial perfusion reserve index was lower in PHT. Structure-function correlations between LVEF and strain versus T2 were noted. T1, ECV were associated with clinical rejection scores. CMR comprehensively evaluates myocardial alterations in PHT.
Introduction
The progressive risk of graft failure in pediatric heart transplantation (PHT) necessitates close surveillance for rejection and cardiac allograft vasculopathy (CAV)1. The current gold standard of surveillance via cardiac catheterization is costly, imperfect and associated with complications2,3. Recent studies have shown that cardiac magnetic resonance imaging (CMR) can provide a compressive assessment of changes in myocardial tissue (T2 mapping: edema, T1-mapping and extracellular volume fraction (ECV): interstitial changes and fibrosis) and cardiac function (CINE derived strain, stress- perfusion). However, limited studies have applied CMR in PHT surveillance 4,5. Our goal was to evaluate the role of a comprehensive CMR in detecting myocardial changes in PHT recipients. We hypothesize that 1) global and regional metrics of cardiac tissue and function are different between PHT and healthy controls, and 2) measures of myocardial tissue abnormalities in PHT are associated with impaired cardiac function (structure-function relationships).Methods
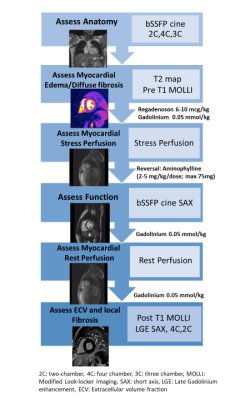
We performed a retrospective review of 26 PHT recipients who underwent comprehensive regadenoson stress perfusion CMR with tissue characterization and compared with 18 age-matched healthy controls. CMR protocol (Figure 1) included left ventricular 2D CINE SSFP imaging, T2-mapping, pre-and post-Gd contrast T1 mapping, myocardial stress perfusion imaging, and late gadolinium enhancement (LGE). Data analysis included left and right ventricular (LV, RV) volumetry and calculation of global and segmental (AHA 16-segment model) T1, T2, and ECV. In addition, qualitative assessment of myocardial stress and rest perfusion was performed with calculation of myocardial perfusion reserve index (MPRI). Finally, LV strain was quantified via feature tracking on CINE SSFP images. Clinical, demographics, rejection score (incorporating all episodes of acute cellular rejection ≥ ISHLT grade 1B or any grade of antibody-mediated rejection) and CAV history were recorded. Correlations between CMR parameters were analyzed using Pearson’s and associations between clinical rejection score and CMR variables were analyzed using logistic regression. P value of <0.05 was considered significant.Results
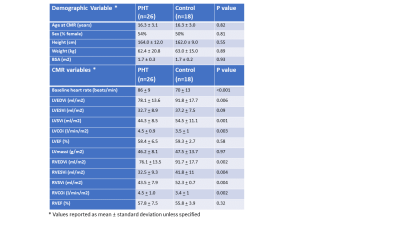
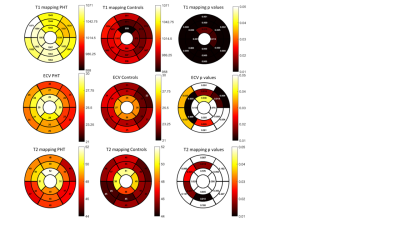
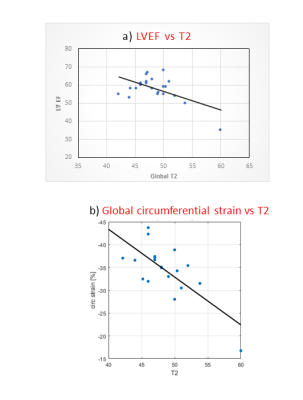
Demographics are reported in Figure 2a. Mean age at transplant was 9.3 + 5.5 years and median duration since transplant was 5.1 years (IQR 7.5 years). One patient had active rejection at the time of CMR, 11/26 (42%) had CAV 1 and 1/26 (4%) had CAV 2 in this PHT cohort with low incidence of rejection or significant CAV. Biventricular volumes were smaller and biventricular cardiac output was higher in PHT (Figure 2b). Global T1 (1053.3 + 41.9ms vs 986.1 + 41.9ms; p<0.001) and ECV (26.5 + 4.0% vs 24.0 + 2.7%, p = 0.017) were higher in PHT compared to controls and global T2 showed a similar trend (48.5 + 3.9ms vs 46.7 + 1.9ms; p = 0.09). Looking at segmental differences based on an AHA 16-segment model, T1 was found to be elevated uniformly in all segments, ECV in 56% and T2 in 25% of segments (Figure 3). Global longitudinal strain (-25.68 + 4.5% vs -25.12 + 2.3%) and circumferential strain (-35.06 + 5.1 % vs -32.87 + 2.1 %) were not significantly different between PHT versus controls respectively. Significant relationships between changes in myocardial tissue structure and function were noted in PHT: increased T2 correlated with reduced LVEF (r = -0.57, p = 0.005), reduced global circumferential strain (r=-0.73, p<0.001) (Figure 4). There were modest associations between rejection score and increased T1 (r2 = 0.27, p = 0.006) and ECV (r2 = 0.38, p = 0.001). During stress testing, no major adverse events were reported and 1/24 (4.1%) PHT had minor adverse events. Although qualitative inducible subendocardial stress perfusion defects were noted only in 3/12 (25%) of those with CAV, the PHT group had a significantly lower global MPRI compared to non-PHT controls (0.69 ± 0.21 vs 0.94 ± 0.22; p < 0.001).Discussion
In a PHT population with low incidence of comorbidities, CMR shows abnormal myocardial alterations compared to non-PHT healthy controls. There were significant relationships between changes in cardiac structure and function noted amongst the PHT, which may point towards underlying pathophysiological mechanisms resulting in functional abnormalities. In addition, with a higher historical burden of rejection, there was worsening myocardial T1 and ECV, reflecting maladaptive myocardial tissue changes. Although perfusion defects were not frequently detected, there was decreased PHT MPRI when compared to controls, suggesting that there are likely abnormalities in the myocardial perfusion reserve even in a seemingly healthy PHT cohort.Conclusion
CMR shows promise in early detection of myocardial alterations in PHT recipients and provides an additional tool for comprehensive graft evaluation. Larger studies are needed to evaluate myocardial structure, function and perfusion changes longitudinally in order to determine the role of CMR in PHT surveillance.Acknowledgements
No acknowledgement found.References
1. Rossano JW, Dipchand AI, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Nineteenth Pediatric Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1185-95.
2. Saraiva F, Matos V, Goncalves L, Antunes M, Providencia LA. Complications of endomyocardial biopsy in heart transplant patients: a retrospective study of 2117 consecutive procedures. Transplant Proc 2011;43:1908-12.
3. Shah KB, Flattery MP, Smallfield MC, et al. Surveillance Endomyocardial Biopsy in the Modern Era Produces Low Diagnostic Yield for Cardiac Allograft Rejection. Transplantation 2015;99:e75-80.
4. Greenway SC, Dallaire F, Kantor PF, et al. Magnetic resonance imaging of the transplanted pediatric heart as a potential predictor of rejection. World J Transplant 2016;6:751-8.
5. Grotenhuis HB, Nyns ECA, Kantor PF, et al. Abnormal Myocardial Contractility After Pediatric Heart Transplantation by Cardiac MRI. Pediatr Cardiol 2017;38:1198-205.
Figures