0963
Feature tracking derived global early diastolic longitudinal strain rate in risk stratification of HFpEF:A preliminary prognostic study1Department of Magnetic Resonance Imaging, Fuwai Hospital & Cardiovascular Institute, Chinese Academy of Medical Sciences & Tsinghua University, Peking Union Medical College, National Center for Cardiovascular Diseases, beijing, China, 2Department of Ultrasound, Fuwai Hospital & Cardiovascular Institute, Chinese Academy of Medical Sciences & Tsinghua University, Peking Union Medical College, National Center for Cardiovascular Diseases, beijing, China
Synopsis
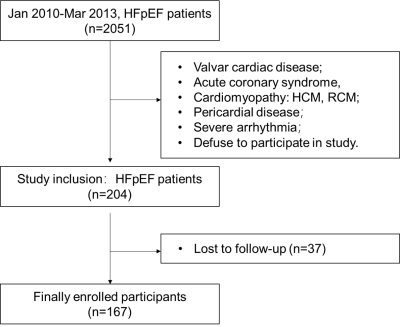
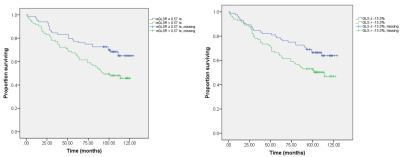
In this retrospective study, 167 HFpEF patients (median age 60 years, 40% women) were included. At a median follow-up of 110 (IQR 104–120) months, 71 patients experienced the primary composite outcome of all-cause death and HF hospitalization. Impaired global early diastolic longitudinal strain rate (eGLSR), defined as an eGLSR < median, 0.57/s, was present in 49.7% of patients and was predictive of the composite outcome (adjusted HR 2.57, 95% CI 1.58–4.45; p=0.001), and HF hospitalization alone (adjusted HR 2.48, 95% CI 1.31–4.70; p=0.005) after adjusting for clinical and conventional imaging variables.
Abstract
INTRODUCTIONImpairment of sub-clinical cardiac function has been described in heart failure with preserved ejection fraction (HFpEF), but its prognostic relevance is not well known. We aimed to use cardiovascular magnetic imaging feature tracking (CMR-FT) to evaluate the prognosis of HFpEF and probably tailor immediate intervention.
METHODS
In this retrospective study, consecutive patients with HFpEF underwent CMR imaging were enrolled from Jan 2010 to Mar 2013. Feature tracking were completed in CMR cine images and three-dimensional strain parameters were measured. Correlations between strain parameters and clinical and imaging variables were analyzed using linear regression. Univariable and multivariable cox proportional regression was performed to determine the prognostic relevance of MRI-derived myocardial strain for primary outcome of heart failure (HF) hospitalizations and all-cause death.
RESULTS
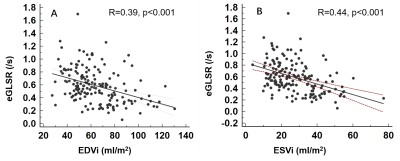
In this study, 167 HFpEF patients (median age 60 years, 40% women) were included. Global early diastolic longitudinal strain rate (eGLSR) was correlated moderately with LVEDVi (r=0.39, p<0.001) and LVESVi (r= 0.44, p<0.001). At a median follow-up of 110 (IQR 104–120) months, 71 patients experienced the primary composite outcome of all-cause death and HF hospitalization. Impaired eGLSR, defined as an eGLSR < median, 0.57/s, was present in 49.7% of patients and was predictive of the composite outcome (adjusted HR 2.57, 95% CI 1.58–4.45; p=0.001), and HF hospitalization alone (adjusted HR 2.48, 95% CI 1.31–4.70; p=0.005) after adjusting for clinical and conventional imaging variables, including age, gender, log transformed NT-proBNP, glomerular filtration rate, comorbidities (coronary artery disease [CAD], atrial fibrillation [AF], hyperlipidemia, pulmonary hypertension, chronic obstructive pulmonary disease), medication (aspirin, statin, angiotensin-converting enzyme inhibitors), LVEF, E/A, E’/A’, left ventricular (LV) end-diastole volume index (EDVi), end-systole volume index (ESVi), LVMi and global longitudinal strain (GLS).
DISCUSSION
There are two key findings in our study. First, CMR-FT technique derived eGLSR was moderately correlated with other imaging variables and can identify different degrees of cardiac dysfunction. Second, eGLSR is a strong risk factor independently associated with the prognosis of HFpEF patients.
Impairment of cardiac systolic and diastolic function in HFpEF patients has been widely proved in multiple studies 1-4, however prognostic study based on cardiac dysfunction is insufficient due to indefinite accepted diagnostic standard of HFpEF and imperfect non-invasive imaging tools. Previous studies have illustrated that strain parameters derived from echocardiography speckle tracking were strong indicator of the prognosis of HFpEF patients 5, and GLS was also included into guidelines6. CMR, as a gold standard for cardiac structure and function illustration, takes advantages in identifying subtle dysfunction via CMR-FT in cine images. As there are no gold reference standard of CMR strain parameters, we choose the median value of eGLSR as cutoff value. HFpEF patients with eGLSR less than the median showed more concomitant CAD, hyperlipidemia, drug medication, and worse cardiac dysfunction. We also found eGLSR was moderately correlated with CMR structural variables, that is, with the increase of EDVi and ESVi, eGLSR was subsequently reduced, indicating impaired cardiac diastolic function. For the first time, our study proposed a new parameter (eGLSR) to distinguish patients with different degrees of cardiac dysfunction.
Several other studies investigated the diagnostic value of FT derived strain parameters 1, 7; the association between NT-proBNP levels 8, focal and diffuse fibrosis 9, and GLS by echocardiography speckle tracking 10 and the prognosis of HFpEF patients. However, the prognostic study of CMR-FT derived strain for HFpEF patients is scant. Up to date now, only one prospective study described the prognostic value of CMR-FT derived strain parameters in HFpEF patients 11. The authors observed GLS was independently associated with of HF hospitalizations and cardiovascular death (HR, 1.06 per 1% strain increase; 95% CI: 1.01, 1.11; P = 0.03) when corrected for risk factors including age, diabetes, renal function, N-terminal pro–b-type natriuretic peptide serum concentration, and right ventricular size and function. However, they didn’t include diastolic parameters and the strain rate is confusing. Our study found eGLSR is a new parameter, strongly associated with the prognosis of HFpEF patients, when adjusting for clinical and imaging parameters in an adequately sized cohort with HFpEF. We may need pay more attention to the strain rate impairment prior to LVEF alterations in clinical practice. And we are now conducting prospective study to further verify this correlation.
CONCLUSION
In participants with heart failure with preserved ejection fraction, eGLSR at cardiovascular MRI was associated with all-cause death and HF hospitalization, and may tailor early intervention.
Acknowledgements
None.References
1. DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, Lin G, Redfield MM, Lewis G, Semigran MJ, Anstrom KJ, Hernandez AF, Velazquez EJ Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail 2017; 19(7):893-900.Jul. doi: 10.1002/ejhf.754.
2. Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014; 63(5):447-56.Feb 11. doi: 10.1016/j.jacc.2013.09.052.
3. Rathi VK, Doyle M, Yamrozik J, Williams RB, Caruppannan K, Truman C, Vido D, Biederman RW Routine evaluation of left ventricular diastolic function by cardiovascular magnetic resonance: a practical approach. J Cardiovasc Magn Reson 2008; 10:36.Jul 8. doi: 10.1186/1532-429x-10-36.
4. Obokata M, Reddy YNV, Borlaug BA Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods. JACC Cardiovascular imaging 2020; 13(1 Pt 2):245-57.Jan. doi: 10.1016/j.jcmg.2018.12.034.
5. Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C, Dokainish H Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography (Mount Kisco, NY) 2015; 32(1):71-8.Jan. doi: 10.1111/echo.12613.
6. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Heart Journal 2019; 40(40):3297-317doi: 10.1093/eurheartj/ehz641.
7. Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, Dawson DK Comprehensive Echocardiographic and Cardiac Magnetic Resonance Evaluation Differentiates Among Heart Failure With Preserved Ejection Fraction Patients, Hypertensive Patients, and Healthy Control Subjects. JACC Cardiovascular imaging 2018; 11(4):577-85.Apr. doi: 10.1016/j.jcmg.2017.05.022.
8. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013; 61(14):1498-506.Apr 9. doi: 10.1016/j.jacc.2012.12.044.
9. Kanagala P, Cheng ASH, Singh A, Khan JN, Gulsin GS, Patel P, Gupta P, Arnold JR, Squire IB, Ng LL, McCann GP Relationship Between Focal and Diffuse Fibrosis Assessed by CMR and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. JACC: Cardiovascular Imaging 2019; 12(11, Part 2):2291-301.2019/11/01/. doi: https://doi.org/10.1016/j.jcmg.2018.11.031.
10. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015; 132(5):402-14.Aug 4. doi: 10.1161/circulationaha.115.015884.
11. Kammerlander AA, Donà C, Nitsche C, Koschutnik M, Schönbauer R, Duca F, Zotter-Tufaro C, Binder C, Aschauer S, Beitzke D, Loewe C, Hengstenberg C, Bonderman D, Mascherbauer J Feature Tracking of Global Longitudinal Strain by Using Cardiovascular MRI Improves Risk Stratification in Heart Failure with Preserved Ejection Fraction. Radiology 2020; 296(2):290-8.Aug. doi: 10.1148/radiol.2020200195.
Figures