0959
Highly Accelerated Cardiac Cine Imaging on a Lower-Field 0.75T MRI1University and ETH Zurich, Zurich, Switzerland
Synopsis
We investigated the feasibility of accelerating cardiac cine imaging on a 0.75T lower-field MRI system by performing retrospective k-t undersampling of cine data at acceleration factors of 3 to 10. Reconstructed images show no unexpected artefacts with nRMSE of 0.6% at 7-fold acceleration. Additionally, the magnetohydrodynamic effect (MHD) in the electrocardiogram was significantly reduced at 0.75T compared to 1.5T promising rapid and robust cine imaging at lower field.
Introduction
Lower-field MRI has the potential to reduce cost while maintaining favorable image quality in cardiac and other applications [1]. Furthermore, lower field strength can reduce magnetic susceptibility induced artefacts [2]. In the present work, we investigated the feasibility of accelerating cardiac cine imaging on a 0.75T lower-field MRI system using total variation compressed sensing reconstruction [3]. Alongside, the magnetohydrodynamic effect in the electrocardiogram-based trigger signal was analysed for 0.75T versus 1.5T to indicate potential improvements of cardiac trigger signal detection.Methods
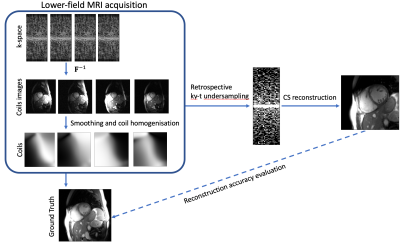
The Carbon-13 channels of the multi-nuclear interface of a 3T Philips Achieva system (Best, The Netherlands) was used to measure proton magnetization at a quarter of the nominal field strength, i.e. at 0.75T. A double Helmholtz volume transmitter and a four-channel receive array (Clinical MR Solutions, Brookfield, WI, USA) were used for excitation and signal reception.Fully-sampled 2D Cartesian cardiac cine imaging was performed at 0.75T using retrospective VCG gating. Relative to the clinical cine protocol at 1.5T, the acquisition bandwidth was lowered to 380 Hz (2.63ms readout duration) to increase signal-to-noise ratio, resulting in a repetition time of 4.2ms, an echo time of 2ms and a flip angle of 60°. Imaging parameters were a square field-of-view of 350mm with 176x176 matrix size yielding an in-plane resolution of approx. 2mm. Slice thickness was chosen to be 10mm and 24 cardiac phases were acquired in short-axis, long-axis and four-chamber view. Per slice orientation a 18s breath hold duration was required.To assess the acceleration potential we performed retrospective undersampling of the k-t space short-axis slide data using variable density sampling [4] masks (see Figure 1). Note that we undersample the acquired k-space data, which is more realistic than resynthesizing the k-space from the fully sampled ground truth image. Sampling density was set proportional to $$$exp(-\frac{|k_x|}{\sigma})$$$. Given the undersampling mask $$$\mathbf{M}$$$, Cartesian Fourier operator $$$\mathbf{F}$$$ and coil sensitivities $$$\mathbf{S}$$$, the compressed sensing reconstruction from the k-t space data $$$\mathbf{d}$$$ is given by the solution of the following vectorial total variation (TV) regularized [5] optimization problem:$$\min_\mathbf{\rho} \|\mathbf{M}\mathbf{F}\mathbf{S}\mathbf{\rho}-\mathbf{d}\|_2^2 + \lambda \|[\alpha\mathbf{\nabla}_x\mathbf{\rho}, \alpha\mathbf{\nabla}_y\mathbf{\rho}, \mathbf{\nabla}_t\mathbf{\rho}]\|_{2,1}$$,where $$$\lambda$$$ is the regularization parameter and the weight $$$\alpha=0.1$$$ balances spatial and temporal image gradients. The optimization is performed numerically using the alternating direction method of multipliers [6] with 5 conjugate gradients iterations for solving inner quadratic optimization. In presented experiments, the regularization parameter $$$\lambda$$$ was chosen to minimize normalized RMSE value averaged over 6 test images for each acceleration rate R.In addition to imaging data and undersampling experiments, VCG signals were recorded in the same subject at 0.75T versus 1.5T to investigate the magnetohydrodynamic (MHD) effect [7].
Results
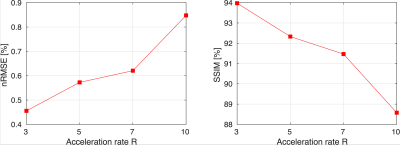
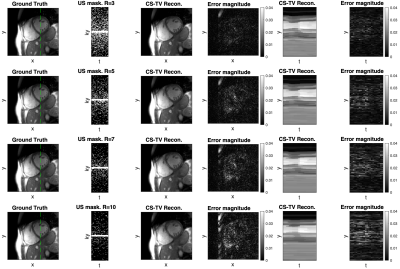
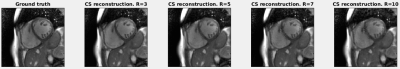
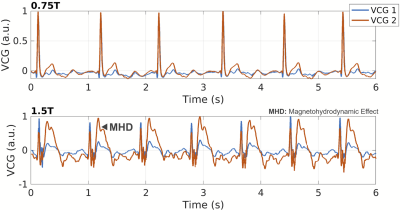
Retrospective undersampling experiments were conducted for acceleration factors R of 3, 5, 7, and 10 with a densely sampled k-space center. Reconstruction accuracy metrics are presented in Figure 2. For acceleration factors of 3–7 nRMSE values are 0.45%-0.62% and the structural similarity index measure (SSIM) is 94%–91.5%, while significant degradation of accuracy is observed for acceleration rate of 10 (nRMSE 0.84% and SSIM 88.6%). Visual analysis of reconstructions from Figure 3 shows that heart motion including papillary muscles can be accurately recovered for acceleration factors up to R=7. For R=10 the y-t profile shows oversmoothing in regions of high motion and apparent staircase artefacts which are typical for total-variation-based regularization. Animated 2D+t short-axis views are presented in Figure 4.In Figure 4, vectorcardiograms (VCGs) are compared between 0.75T (top) and 1.5T (bottom) of the same volunteer. At 0.75T, the magnetohydrodynamic effect (MHD) is significantly reduced.
Discussion
We have demonstrated the feasibility of up to 10-fold accelerated cardiac cine MRI at 0.75T using a four-channel receive array. Reconstructed images are considered diagnostic, with accuracy vs. acceleration tradeoff being comparable to standard MRI at 1.5 and 3 T. At 10-fold acceleration considerable image degradation was noted which may be addressed by employing more advanced reconstruction methods based on dictionary learning [8] or variational networks [9,10]. We anticipate further accuracy improvements for systems with a body coil, as assumptions of current coil calibration method (Figure 1) are violated in the presence of large motion.Furthermore, the reduced magnetohydrodynamic effect at 0.75T versus 1.5T promises improved robustness of cardiac triggering and potentially offers simplification of the setup by relying on a single-channel electrocardiogram in the future.
Acknowledgements
No acknowledgement found.References
[1] Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019;293(2):384-393. doi:10.1148/radiol.2019190452
[2] Campbell-Washburn AE, Jiang Y, Körzdörfer G, Nittka M, Griswold MA. Feasibility of MR fingerprinting using a high-performance 0.55T MRI system. In: Intl. Soc. Mag. Reson. Med. 28. ; 2020:0868.
[3] Lustig M, Donoho DL, Santos JM, Pauly JM. Compressed sensing MRI. IEEE signal processing magazine. 2008 Mar 21;25(2):72-82.
[4] Johannes Schmidt, Claudio Santelli, and Sebastian Kozerke. Optimized k-t sampling for combined parallel imaging and compressed sensing reconstruction. Proc. Intl. Soc. Mag. Reson. Med. 22, 2014.
[5] Blomgren P, Chan TF. Color TV: total variation methods for restoration of vector-valued images. IEEE transactions on image processing. 1998 Mar.
[6] Boyd S, Parikh N, Chu E. Distributed optimization and statistical learning via the alternating direction method of multipliers. Now publishers Inc; 2011.
[7] Fischer SE, Wickline SA, Lorenz CH. Novel real‐time R‐wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magnetic resonance in medicine. 1999 Aug;42(2):361-70.
[8] Caballero, Jose & Price, Anthony & Rueckert, Daniel & Hajnal, Joseph. (2014). Dictionary learning and time sparsity for dynamic MR data reconstruction. IEEE Transactions on medical imaging. 33. 979-994.
[9] Hammernik K, Klatzer T, Kobler E, Recht MP, Sodickson DK, Pock T, Knoll F. Learning a variational network for reconstruction of accelerated MRI data. Magnetic resonance in medicine. 2018 Jun;79(6):3055-71.
[10] Vishnevskiy V, Walheim J, Kozerke S. Deep variational network for rapid 4D flow MRI reconstruction. Nature machine intelligence. 2020 Apr;2(4):228-35.
Figures