0957
1D-CNN for the Detection of IDH and TERTp Mutations in Diffuse Gliomas using Proton Magnetic Resonance Spectroscopy1Institute of Biomedical Engineering, Bogazici University, Istanbul, Turkey, 2Department of Medical Pathology, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 3Center for Neuroradiological Applications and Reseach, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 4Department of Molecular Biology and Genetics, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 5Department of Neurosurgery, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey, 6Department of Radiology, Acıbadem Mehmet Ali Aydinlar University, Istanbul, Turkey
Synopsis
Isocitrate dehydrogenase (IDH) and telomerase reverse transcriptase promoter (TERTp) mutations affect the clinical behavior and survival rate of diffuse gliomas. The detection of these mutations preoperatively is very critical for treatment planning. In this study, three different one dimensional convolutional neural network (1D-CNN) models were designed to identify IDH mutant (IDH-mut), TERTp mutant (TERTp-mut), and TERTp-only (IDH-wild type and TERTp-mut) gliomas based on proton magnetic-resonance spectroscopy (1H-MRS). The 1D-CNN models could identify IDH-mut, TERTp-mut, and TERTp-only gliomas with 94.11%, 76.92%, and 82.05% accuracies, respectively. This study showed the potential of deep-learning in predicting especially IDH-mutations in gliomas using 1H-MRS data.
Introduction
Isocitrate dehydrogenase (IDH) and telomerase reverse transcriptase promoter (TERTp) mutations cause gliomas to have different clinical behavior and survival rate [1-3]. While IDH mutant (IDH-mut) gliomas have better treatment response and overall survival rate, IDH wildtype TERTp mutant (TERTp-only) gliomas have been reported to have the worst overall survival in both low- and high-grade gliomas [1]. Preoperative and noninvasive detection of these mutations has been drawing great interest. Proton magnetic resonance spectroscopy (1H-MRS) has been successfully applied for the detection of IDH and TERTp mutations [4-7]. This study aims to develop an end-to-end classifier for the identification of IDH-mut, TERTp-mut, and TERTp-only gliomas based on one dimensional convolutional neural network (1D-CNN) architecture.Methods
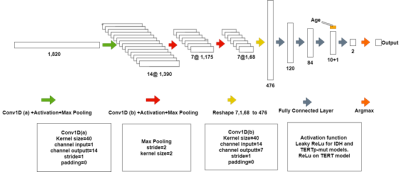
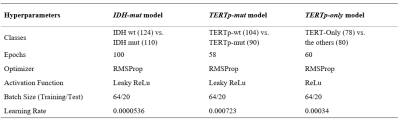
237 glioma patients (142M/95F, mean age: 44.68±14.11 years, range: 20-84 years) were included in this study. The patients were scanned before surgery at a 3T Siemens Prisma scanner (Erlangen, Germany) using a 32-channel head coil. The brain tumor protocol included pre- and post-contrast (gadolinium DTPA) T1-weighted TSE (TR=500 ms, TE=10 ms), T2-weighted TSE (TR=5000 ms, TE=105 ms), and T2*-weighted gradient-echo echo-planar imaging (EPI) dynamic susceptibility contrast (DSC) MRI (TR=1500 ms, TE=30 ms). 1H-MRS data were acquired from the solid tumor region excluding necrosis, edema, and hemorrhage using a Point Resolved Spectroscopy (PRESS) sequence (TR/TE=2000/30 ms, voxel size=1-8 cm3). LCModel spectral fitting program [8] was used for quantification of MRS data and fitted spectrums were used in the glioma classification. TERTp and IDH1 or IDH2 (IDH1/2) mutations in the tissue were determined by either minisequencing or Sanger sequencing. Preprocessing on input data was performed before the classification using L2 normalization followed by smoothing (Savitzky-Golay filter with a window size of 11 and an order of 2) followed by Yeo-Johnson power transformation and min-max normalization [9]. The data was split into training, test, and validation sets. Three separate models were developed for the identification of IDH-mut, TERTp-mut and TERTp-only gliomas using the same architecture, which is shown in Figure 1. Cross-entropy loss was used during the training of the models. Optuna [10] was employed for hyperparameter tuning of the models with 50 trials and the optimized parameters are shown in Table 1. ADASYN was used to overcome the imbalanced dataset problem for the TERTp-only model [11]. All the computations were performed in Python.Results
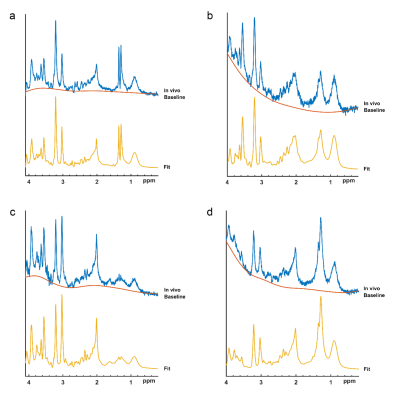
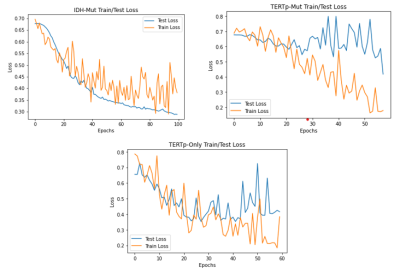
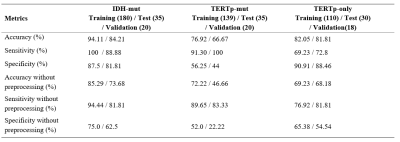
Figure 2 shows example spectra for (a) an IDH-mut&TERTp-mut, (b) an IDH-mut&TERTp-wt, (c) a TERTp-only, and (d) an IDH-wt&TERTp-wt glioma. Table 2 shows the main performance metrics of the 1D-CNN models for both test and validation sets with/without preprocessing. For the IDH-mut model, 94.11% accuracy was obtained with a sensitivity of 100% and a specificity of 88.88% on the test set, while 84.21% accuracy was achieved with a sensitivity of 87.5% and with a specificity of 81.81% on the validation set. TERTp-mut model could detect TERTp mutation in gliomas with an accuracy of 76.92% (sensitivity:91.30%, specificity:56.25%) on the test set and an accuracy of 66.67% (sensitivity:100%, specificity:44%) on the validation set. For the detection of TERTp-only gliomas, the model achieved 82.05% accuracy with a sensitivity of 69.23% and a specificity of 90.91% on the test set and 81.81% accuracy with a sensitivity of 72.8% and a specificity of 88.46% on the validation set. Preprocessing improved the classification results for all the models. The training and test losses of these models are shown in Figure 2.Discussion and Conclusion
The results of this study indicated that IDH and TERTp mutations in gliomas could be detected accurately using 1H-MRS and deep-learning methodology. Although deep learning models have been previously used to detect the mutations of gliomas on anatomical MR images [12, 13], this study showed their potential in predicting mutations in gliomas using 1H-MRS data. Predicting molecular subgroup of gliomas using deep-learning models instead of machine learning algorithms removed the need for feature selection before the classification. Moreover, using the whole 1H-MRS data instead of metabolite concentrations might have better taken into account all the metabolite contributions, especially the overlapping peaks that are harder to separately quantify.Acknowledgements
This study has been supported by TUBITAK 1003 grant 216S432.References
1.Eckel-Passow, J.E., et al., Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med, 2015. 372(26): p. 2499-508.
2.Ogura, R., et al., Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology, 2015. 35(4): p. 324-35.
3.Takano, S., et al., Immunohistochemistry on IDH 1/2, ATRX, p53 and Ki-67 substitute molecular genetic testing and predict patient prognosis in grade III adult diffuse gliomas. Brain Tumor Pathol, 2016. 33(2): p. 107-16.
4.Ozturk-Isik, E., et al., Identification of IDH and TERTp mutation status using 1H‐MRS in 112 hemispheric diffuse gliomas. Journal of Magnetic Resonance Imaging, 2019. 51.
5.Andronesi, O.C., et al., Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med, 2012. 4(116): p. 116ra4.
6.Choi, C., et al., 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med, 2012. 18(4): p. 624-9.
7.Nagashima, H., et al., Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol, 2016. 18(11): p. 1559-1568.
8.Provencher, S.W., Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed, 2001. 14(4): p. 260-4.
9.Engel, J., et al., Breaking with trends in pre-processing? TrAC Trends in Analytical Chemistry, 2013. 50: p. 96-106.
10.Akiba, T., et al., Optuna: A Next-generation Hyperparameter Optimization Framework, in Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining. 2019, Association for Computing Machinery: Anchorage, AK, USA. p. 2623–2631.
11.Haibo, H., et al. ADASYN: Adaptive synthetic sampling approach for imbalanced learning. in 2008 IEEE International Joint Conference on Neural Networks (IEEE World Congress on Computational Intelligence). 2008.
12.Bangalore Yogananda, C.G., et al., A novel fully automated MRI-based deep-learning method for classification of IDH mutation status in brain gliomas. Neuro-Oncology, 2020. 22(3): p. 402-411.
13.Chang, P., et al., Deep-Learning Convolutional Neural Networks Accurately Classify Genetic Mutations in Gliomas. American Journal of Neuroradiology, 2018. 39(7): p. 1201.
Figures