0955
Early detection of radiation-induced injury and prediction of cognitive deficit by MRS metabolites in radiotherapy of low grade glioma1Isfahan University of Medical Sciences, Isfahan, Iran (Islamic Republic of), 2Bushehr University of Medical Sciences, Bushehr, Iran (Islamic Republic of), 3Tehran University of Medical Sciences, Tehran, Iran (Islamic Republic of), 4Milad Hospital, Isfahan, Iran (Islamic Republic of)
Synopsis
To compare the sensitivity of alteration in MRS metabolites and MoCA and ACE-R cognitive tests in early and early-delayed post-radiation phases in detection of radiation-induced injury of Low Grade Glioam patients. The MRS metabolites, the ACE-R and MoCA, and the dosimetric parameters in the corpus callosum were analyzed during RT and up to 6-month post-RT for 10 LGG patients. NAA/Cr and Cho/Cr declined significantly at least 3 months before detecting alterations in ACE and MoCA cognitive tests. Therefore, the MRS-based biomarkers may be more sensitive than the state of the art cognitive test tools in prediction of post-radiation cognitive impairments.
Introduction
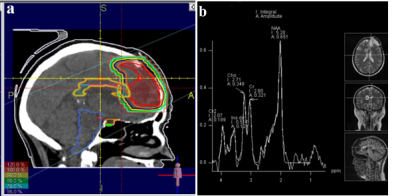
Radiation therapy (RT) plays a major role in treatment of low grade glioma (LGG) patients1. During RT, the brain normal tissue will inevitably be exposed to the radiation, causing radiation-induced brain injury (RIBI), classified as early, early-delayed, and late-delayed2,3. MR spectroscopy (MRS) can evaluate RIBI by assessing the metabolic concentrations of N-Acetylaspartate (NAA), Choline (Cho), and Creatine (Cr) 4,5. The corpus callosum (CC) is evidenced to be sensitive to the radiation effects leading cognitive decline6-8. Post-RT cognitive impairments are not commonly detectable by cognitive assessment tools such as the ACE, and MoCA in early-delayed phase9-11. This study aimed to investigate whether MRS metabolite variations can be a more sensitive tool for detecting RIBI in early post-radiation phase compared to conventional cognitive tools.Methods
10 patients histologically confirmed with low grade glioma underwent craniotomy and RT without receiving chemotherapy. Standard 3D conformal radiotherapy was prescribed using 1.8 Gy in 30 fractions by ONCOR linear accelerators. All patients had MRI/MRS scans at the baseline, at the fourth week of RT, 1, 3 and 6-month after RT. The Farsi versions of the (MoCA-P) and (ACE-P) were completed by all patients at the time of their MRI scans. ACE-R includes attention/ orientation, memory, fluency, language, and visuospatial ability. MoCA-P contains visuospatial; naming; memory; language; abstraction; and attention domains. The significance of sequential deviations from the baseline values for the metabolic ratios of Cho/ Cr and NAA/Cr; and the ACE-R and MoCA results were inspected using a paired-sample Student’s t test. All tests were two-tailed and conducted at the 5% significance level. The correlations between the metabolic ratios and ACE-R and MoCA scores were assessed by Pearson correlations. The correlation between the alterations in NAA/Cr and Cho/Cr in with the alterations in ACE-R and MoCA scores in consecutive time points was also inspected.Results
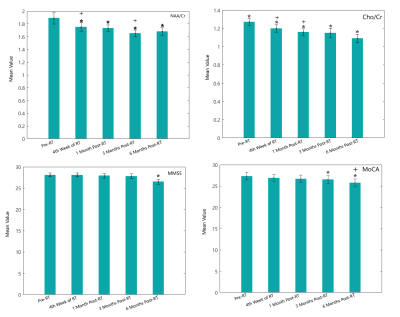
Compared to the pre-RT values, NAA/Cr ratio decreased by 0.10±0.03 (P = 0.001), 0.12±0.05 (P = 0.04), 0.17±0.05 (P = 0.001) and 0.18±0.06 (P = 0.005) at the fourth week of RT, 1-month, 3-month and 6-month post- RT, respectively. Again compared to the pre-RT values, the mean of Cho/Cr ratio decreased by 0.12±0.05 (P = 0.0001), 0.15±0.03 (P = 0.0001), 0.24±0.06 (P = 0. 04), and 0.20±0.07 (P = 0.003) at the fourth week of RT, 1-month, 3-month and 6-month post-RT, respectively. Compared to the baseline values, both the MoCA and ACE-R scores taken 6-month post RT were statistically significant (P=0.0001 and 0.002). Overall, five and four patients had ACE-R and MoCA scores below the normal at 6-month post-RT, respectively. A significance decline was observed in MoCA language domain at 6-month compared to 3-month post-RT (P-value = 0.05). Also, there was a significant difference between MoCA language score at the 6-monnh follow up compared to the baseline value (P-Value=0.045). Furthermore, a significant decrease was seen between the score of MoCA memory subdomain at the 6-month post-RT to the baseline value (P-value= 0.04). ACE-R showed declines in language and verbal fluency subdomains for two patients at the 4th week of RT. However, both ACE-R and MoCA showed decline by both the language and verbal fluency subdomains at 6-month post-RT. Overall, ACE-R and MoCA tests showed cognitive declines for 6 patients, 6-month post-RT.Discussion
Significant unremitting decline from fourth week of RT up to 6-month post-RT in NAA/Cr and Cho/Cr ratio confirmed the hypothesis that the microstructural metabolic changes in CC can predict cognitive declines at the early-delayed phase12. Significant changes in patient’s MoCA and ACE-R scores at 3 and 6-month post-RT were observed, while abnormal score was reported for for patients with lower baseline scores, similar to the Brown et al. study13. A decline in language and verbal fluency, visuospatial and memory scores specifically at 3 and 6-month post-RT was demonstrated. The variation in the microstructure of the CC can strongly affect visuospatial perception and verbal fluency tasks, as well as other cognitive functions such as memory which may result in serious cognitive disorders 14-16. Mean delivered dose to the critical structures as one of the effective parameters on the white matter and consequently cognitive impairments showed a negative correlation with the deviation of NAA/Cr between pre-RT and 4th week of RT, and a negative correlation with the deviation of NAA/Cr between 4th week of RT and 1month post-RT. The dose dependence of the metabolic deviance in normal appearing brain tissue is a controversial issue confirmed by some studies, yet rejected by others17.Conclusion
Significant variation of MRS metabolites in the corpus callosum started at early phases after RT of LGG patients up to early-delayed and delayed phases. Since the corpus callosum plays a critical role in most cognitive pathways, such early-delayed variation of metabolites can be considered a more sensitive predictor of long term detrimental effects of radiotherapy on cognitive functions, in comparison with ACE-R or MoCA. This study suggests that the MRS study of early variations in NAA/Cr and Cho/Cr in the corpus callosum may be exploited to modify the treatment plans for the patients and to design more sensitive cognitive assessment tools and introduce reliable quantitative imaging biomarkers that can predict cognitive decline in LGG patients before it manifests at the late-delayed stages.Acknowledgements
No acknowledgement found.References
1. Balentova S, Adamkov M. Molecular, cellular and functional effects of radiation-induced brain injury: a review. Int. J. Mol. Sci.. 2015;16(11):27796-815.
2. Robbins M, Greene-Schloesser D, Peiffer AM, Shaw E, Chan MD, Wheeler KT. Radiation-induced brain injury: A review. Front. Oncol.. 2012;2:73.
3. Béhin A, Delattre J-Y, editors. Complications of radiation therapy on the brain and spinal cord. Seminars in neurology; 2004.
4. Connor M, Karunamuni R, et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother Oncol. 2017;123(2):209-17.
5. Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki L. Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol. 2010;48(2):81-92.
6. Di Paola M, Spalletta G, Caltagirone C. In vivo structural neuroanatomy of corpus callosum in Alzheimer's disease and mild cognitive impairment using different MRI techniques: a review. J Alzheimers Dis. 2010;20(1):67-95.
7. Thomann PA, Wüstenberg T, Pantel J, Essig M, Schröder J. Structural changes of the corpus callosum in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(4):215-20.
8. Yamauchi H, Fukuyama H, Shio H. Corpus callosum atrophy in patients with leukoaraiosis may indicate global cognitive impairment. Stroke. 2000;31(7):1515-20.
9. Fu X, Shrestha S, Sun M, Wu Q, Luo Y, Zhang X, et al. Microstructural White Matter Alterations in Mild Cognitive Impairment and Alzheimer’s Disease. Clin. Neuroradiol. 2019.
10. Luo C, Li M, Qin R, Chen H, Yang D, Huang L, et al. White Matter Microstructural Damage as an Early Sign of Subjective Cognitive Decline. Front. Aging Neurosci. 2020;11(378).
11.Zhang M, Jin H, Sun S, Bu M, Su Q, Liu G, et al. Detection of radiation brain injury of malignant glioma by 1H-MRS. J. of Jilin University (Med Edition). 2011;37(4):742-5.
12. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: A review. Frontiers in oncology. 2012;2:73-.
13. Brown PD, Buckner JC, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J. Clin. Oncol.2003;21(13):2519-24.
14. Giorgio A, De Stefano N. Cognition in multiple sclerosis: relevance of lesions, brain atrophy and proton MR spectroscopy. Neurol. Sci. 2010;31(2):245-8.
15. Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, et al. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain and cognition. 2008;67(2):225-33.
16. Kozlovskiy S, Vartanov A, Pyasik M, Nikonova E. Functional role of corpus callosum regions in human memory functioning.Int. J. Psychophysiol. 2012;3(85):396-7.
17. Sundgren P. MR spectroscopy in radiation injury. Am. J. Neuroradiol. 2009;30(8):1469-76.
Figures