0953
Correlation between Amide Proton Transfer Imaging and Pathological Staging of Rectal Cancer1Medical Imaging Center, Nanfang Hospital, Southern Medical University, Guangzhou, China, 2Intervention, Traditional Chinese Medicine Hospital of Guangdong Province, Guangzhou, China, 3Philips Healthcare, Guangzhou, China
Synopsis
Rectal cancer is the third deadliest cancer in the world. The choice of its clinical treatment plan is closely related to clinical staging, which mainly depends on preoperative MR staging. Clinical work experience has found that the MR staging obtained by the existing MR technology is often less precise and often higher than the postoperative pathological staging. In order to solve this problem, our research has found that Amide Proton Transfer(ATP) imaging has a strong correlation with postoperative pathological staging, which can help clinicians to make a more accurate diagnosis of rectal cancer staging before surgery.
Purpose
Colorectal cancer is the third deadliest cancer in the world, with more than one million people dying from the disease each year.1 The current treatment for this disease is divided into surgical treatment and neoadjuvant therapy (preoperative radiotherapy and chemotherapy). According to the latest clinical application guidelines for rectal cancer, MR examination plays a crucial role in the clinical staging of tumors and facilitates the selection of the best treatment plan for patients. However, in clinical work, we find that MR clinical staging results often differ from postoperative pathological staging results, and usually the MR staging grade is higher. In order to stage rectal cancer optimally before surgery, new techniques are expected. In recent years, Amide Proton Transfer(ATP) imaging has emerged as a powerful MR imaging technique that can assess protein and peptide levels non-invasively.2 Because of protein overexpression in tumors, ATP imaging has significant advantages in tumor diagnosis and identification. Xu et al.3 demonstrated APT imaging in glioma grading and IDH mutation status prediction has significant significance. ATP imaging has been shown to be significant in the grading and prognostic assessment of a variety of tumors, including hepatocellular carcinoma4-5 and nasopharyngeal carcinoma.6 However, there are few reports on ATP imaging in rectal cancer, especially studies on the correlation between ATP imaging and pathological staging have not been reported. Therefore, the purpose of this experiment was to explore the correlation between ATP imaging and pathological staging.Methods
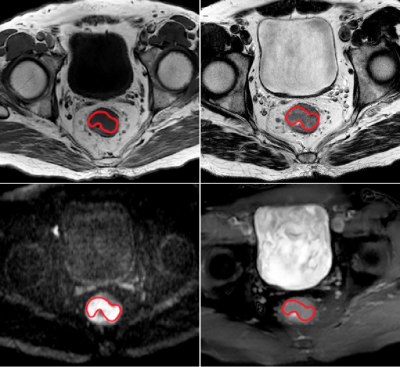
Subjects and data acquisition: MRI examination including ATP imaging was conducted on 33 rectal cancer patients, who were pathologically confirmed to have adenocarcinoma by endoscopic biopsy prior to MR examination. The specific inclusion criteria are shown in Figure 1. All MRI examinations were performed with a Philips Achieva 3T MR scanner (Philips Healthcare Inc, Best, The Netherlands) with a 16-channel SENSE-XL-Torso coil. Conventional TIWI, T2WI and DWI sequences were first performed, and then the largest cross-section of the tumor was selected for ATP imaging before the patient was injected with the contrast agent required for the enhancement scan, and the parameters associated with the ATP sequence were as follows: repetition time(TR) = 3274ms, echo time (TE) = 5.9ms, field of view(FOV) = 230×355mm2, voxel size = 0.82×0.82×5mm3, matrix size = 128×194, thickness=5mm.Data analysis: The first step is to perform APT image processing using MATLAB (MathWorks, Inc., Natick, MA, USA) program. z Spectral analysis, minimization of field inhomogeneity and reproducibility analysis were performed with reference to several literature for detailed methods.7-8 In the second step, the primary tumor contours were manually drawn on the APT images using Image J(National Institutes of Health, Baltimore, MD, USA) with reference to other MR sequential imaging and excluding hemorrhages, vessels and necrosis evident on the anatomical images. The mean value of APT asymmetry (APTmean) was extracted for statistical analysis. To assess interobserver and interobserver agreement, tumor contouring was performed by 2 experienced radiologists (with at least 10 years of experience) each.
Statistical analysis: Statistical analysis of all data was performed by SPSS 25 software package. The result of p-values less than 0.05 were considered to be statistically different. Independent samples t-test or Mann-Whitney U test was used for statistical analysis of dichotomous variables. The statistical method used for tumor location and histological grade was the Kruskal-Wallis H test. Receiver operating characteristic curves (ROC) were used to assess the diagnostic performance of ATP mean values for pathological staging.
Results
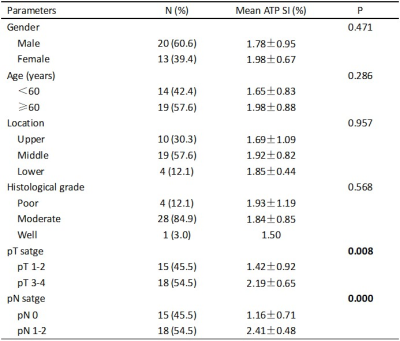
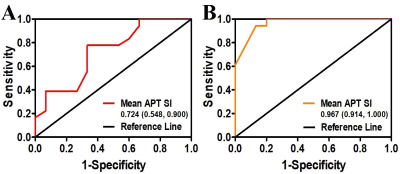
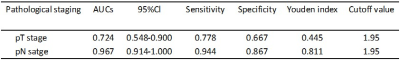
Table1 shows the correlations between mean ATP SI(%) with clinicopathologic characteristics in detail. Both T-stage and N-stage were divided into two categories based on pathological findings: low-grade group (T1-2) and high-grade group (T3-4); metastatic lymph node-negative group (N0) and metastatic lymph node-positive group (N1-2). pT3-4 group had significantly higher mean ATP SI(%) values than pT1-2 group(p=0.008). The mean ATP SI(%) values in the pN1-2 group were also significantly different from those in the pN0 group(p=0.000). ROC curve analysis (Figure 3, Table 2) indicated that the ATP mean SI(%) had good diagnostic performance in distinguishing pathological T and N stages of rectal cancer, especially in N stage. The AUC value of ATP mean SI(%) in predicting pT stage and pN stage of rectal cancer were 0.724 and o.967, respectively.Discussion and conclusion
This is the first study to report the correlation between amide proton transfer imaging and pathological staging of rectal cancer. The results of this study showed that the ATP mean value could well predict pathological T and N stages.Therefore, this technique can be clinically applied in combination with conventional MR imaging sequences (T1WI, T2WI, DWI, etc.) to achieve a better preoperative clinical staging and provide a more adequate and favorable evidence for the selection of the best treatment plan for rectal cancer patientsAcknowledgements
We thank the Philips Healthcare team for their technical support for this study.References
1. Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R. L.; Torre, L. A.; Jemal, A., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68, (6), 394-424.
2. Zhou, J.; Blakeley, J. O.; Hua, J.; Kim, M.; Laterra, J.; Pomper, M. G.; van Zijl, P. C., Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 2008, 60, (4), 842-849.
3. Xu, Z.; Ke, C.; Liu, J.; Xu, S.; Han, L.; Yang, Y.; Qian, L.; Liu, X.; Zheng, H.; Lv, X.; Wu, Y., Diagnostic performance between MR amide proton transfer (APT) and diffusion kurtosis imaging (DKI) in glioma grading and IDH mutation status prediction at 3T. Eur J Radiol 2020, 134, 109466.
4. Wu, B.; Jia, F.; Li, X.; Li, L.; Wang, K.; Han, D., Comparative Study of Amide Proton Transfer Imaging and Intravoxel Incoherent Motion Imaging for Predicting Histologic Grade of Hepatocellular Carcinoma. Front Oncol 2020, 10, 562049.
5. Wu, B.; Jia, F.; Li, X.; Zhang, M.; Han, D.; Jia, Z., Amide Proton Transfer Imaging vs Diffusion Kurtosis Imaging for Predicting Histological Grade of Hepatocellular Carcinoma. J Hepatocell Carcinoma 2020, 7, 159-168.
6. Qamar, S.; King, A. D.; Ai, Q. H.; Mo, F. K. F.; Chen, W.; Poon, D. M. C.; Tong, M.; Ma, B. B.; Yeung, D. K.; Wang, Y.; Yuan, J., Pre-treatment amide proton transfer imaging predicts treatment outcome in nasopharyngeal carcinoma. European radiology 2020, 30, (11), 6339-6347.
7. Nishie, A.; Takayama, Y.; Asayama, Y.; Ishigami, K.; Ushijima, Y.; Okamoto, D.; Fujita, N.; Tsurumaru, D.; Togao, O.; Manabe, T.; Oki, E.; Kubo, Y.; Hida, T.; Hirahashi-Fujiwara, M.; Keupp, J.; Honda, H., Amide proton transfer imaging can predict tumor grade in rectal cancer. Magnetic resonance imaging 2018, 51, 96-103.
8. Nishie, A.; Asayama, Y.; Ishigami, K.; Ushijima, Y.; Takayama, Y.; Okamoto, D.; Fujita, N.; Tsurumaru, D.; Togao, O.; Sagiyama, K.; Manabe, T.; Oki, E.; Kubo, Y.; Hida, T.; Hirahashi-Fujiwara, M.; Keupp, J.; Honda, H., Amide proton transfer imaging to predict tumor response to neoadjuvant chemotherapy in locally advanced rectal cancer. J Gastroenterol Hepatol 2019, 34, (1), 140-146.
Figures