0951
Preoperative discrimination between the low-grade glioma and high-grade glioma and early exploration of metastatic margin with APT imaging at 7T1Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, China, 2MR Collaboration, Siemens Healthcare Ltd., Shanghai, China, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
Synopsis
Preoperative assessment of histological grades and possible margin of glioma are challenges to neurosurgeons, which may also make a huge difference in therapeutic strategies. In this study, we aim to establish a grade-discrimination criterion via APT and explore the possible metastatic margin of diffuse glioma at 7T. Eleven patients underwent structural imaging and APT CEST imaging. Results of the APT were merged with the T1 image, which showed a promising indication of preoperative histological grades. Meanwhile, with the early exploration of metastatic margin, the APT CEST at 7 Tesla may significantly facilitate the surgical strategy and surgical excision extension.
Introduction
Glioma is the most common malignant intradural tumor, with an incidence of about 6 per 100,0001. WHO grade IV glioblastoma accounts for more than 50% of all gliomas and has the highest degree of malignancy. The median survival time after surgical resection combined with radiotherapy and chemotherapy is only 14-16 months. After treatment with tumor electric field, the median survival time only improves to 20.1 months2. How to improve the treatment effect of glioma is an eternal topic for multidisciplinary clinicians such as neurosurgeons, oncologists, and radiologists. Despite the constant developing treatment, surgery is the effective way and the most critical step in the comprehensive treatment of gliomas. It is important to establish a good preoperative diagnosis and boundary determination for guiding surgery. Chemical Exchange Saturation Transfer (CEST) of amide protons has been increasingly employed for tumor grading3. In this study, we attempt to use the 7T to differentiate LGG (WHO Grade I and II) from HGG (WHO Grade III and IV) and to explore the possible metastatic margin, combining neuro-navigational system to guiding the surgical strategy.Material and methods
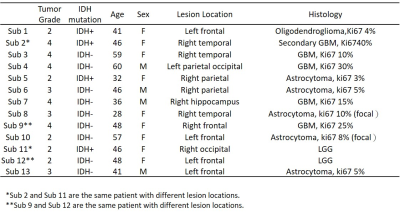
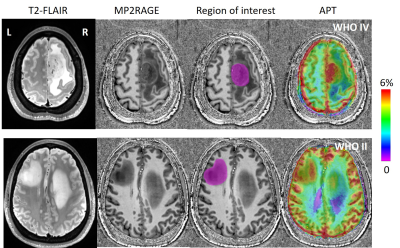
The study was approved by the local institutional ethics committee, and written informed consent was obtained from all patients. A total of eleven patients (seven females, four males; median age, 46 years; age range, 28–60 years; see Table 1) were recruited with the inclusion criteria of (1) a suspected intracranial occupying lesion on routine MRI; (2) had surgical resection with histological examination. All of the patients underwent surgical biopsy or resection for definitive histopathologic diagnoses. All patients were scanned at a 7 T MRI scanner (MAGNETOM Terra, Siemens Healthineers, Erlangen, Germany). MP2RAGE (TR=3800 ms, TI1=800ms, TI2=2700ms, TE=2.29ms, FA=7°,and resolution=0.7mm isotropic) and T2-Flair (TR=9000ms, TI=2600ms, TE=274ms, FA=120°, and resolution=0.8 mm isotropic) were acquired. The APT CEST data (TR=3.4ms, TE=1.59ms, FA=6°,and resolution=1.6mm x 1.6mm x 5mm, 56 RF offsets, B1=0.6, 0.75, 0.9mT) were calculated using the Lorentzian fit method4 with B1 correction. Region of interest (ROI) was drawn on T1-weighted axial slices within the glioma lesion (Figure 1). The 90th percentile of the APT-CEST distribution within each patient's ROI was used for statistical analysis.Results
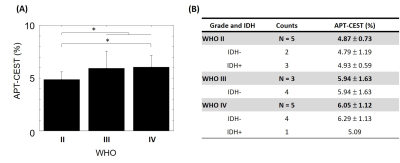
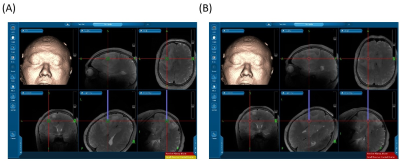
Figure 1 shows the T2-Flair images, T1 images, ROIs, and APT in a patient. There were two tumor lesions with different grades (The top row is from WHO Grade IV, and the bottom row is from WHO Grade II). Larger APT values can be found in the high-grade region (5.63%) compare to high-grade region (6.29%). The mean and standard deviation of the APT among all patients were calculated in WHO grade II, grade III, and grade IV: 4.87 ± 0.73, 5.94 ± 1.63, and 6.05 ± 1.12. The APT-CEST signal increased when the WHO grade was higher. In Figure 2A, the APT-CEST signal was significantly higher in HGG than in LGG (p-value <0.05). The IDH mutation status (a significant molecular biomarker5 of predicting the prognosis) in each patient was classified by WHO (Figure 2B). The APT-CEST signal of patients without and with IDH mutation were respectively 4.79 ± 1.19 and 4.93 ± 0.59 in WHO grade II, and 6.29 ± 1.13 and 5.09 ± n/a in WHO grade IV. There were two biopsy locations in subject 15 in the margin and core of the lesion in left frontal (the biopsy path is shown in Figure 3). The pathology of the first location of biopsy (figure 3A) is gliosis with mild abnormal in nuclear with a pathologic diagnose as possible low-grade glioma; while the second biopsy position (figure 3B) was diagnosed as anaplastic astrocytoma (WHO grade III). The degradation of pathologic features shown there may exist a possible metastatic margin in the edema of glioma especially in HGG, which could be a revolution of the strategy to glioma surgery:” Metabolic Resection”.Discussion and conclusion
Higher APT-CEST signal in HGG compared to LGG was found. Finding the edge lesion can be challenging. The edema in HGG can obscure the ROI definition, which can cause larger deviation. We use 90th percentile data within the ROI to reduce this problem. The mean APT-CEST signal is lower in tumors with mutations than without IDH mutation in HGG group (Figure 3B). However, more patients need to be recruited to reveal the correlation between WHO and IDH mutation status. Besides the preoperative grade prediction, for those patients without lesion-enhancement in Gd-enhanced T1 image, the resection margin is a dilemma for neurosurgeon. For the sake of enhancing the survival time of patient, a radical resection of the lesion and adjacent edema even normal brain tissue is obviously a right choice; meanwhile, as every brain region presents a specific function, the radical surgery strategy will hugely damage the normal function even sacrifice patients’ living quality. Thus, a new concept of resection margin: metabolic margin may reach the balance of maximum resection and function saving. In this case, we did a small exploration of multimodality neuro-navigational guided biopsy, trying to expose the possible margin and furtherly confirm that. In our next step, we will recruit more patients to modify our preoperative metabolic margin and make efforts to reach the maximum resection with function ensuring via the aids of APT CEST imaging 7T.Acknowledgements
No acknowledgement found.References
1. Holdhoff M. “Role of Molecular Pathology in the Treatment of Anaplastic Gliomas and Glioblastomas.” J Natl Compr Canc Netw. 2018;16(5S):642-645.
2. NCCN, NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers (Version 3.2019). 2019.
3. Zhou, J., et al., “Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging.” Magn Reson Med, 2008. 60(4): p. 842-9.
4. Windschuh, Johannes, et al. "Correction of B1‐inhomogeneities for relaxation‐compensated CEST imaging at 7 T." NMR in biomedicine 28.5 (2015): 529-537.
5. Waitkus MS, Diplas BH, Yan H. “Biological Role and Therapeutic Potential of IDH Mutations in Cancer.” Cancer Cell. 2018;34(2):186-195.
Figures