0942
Assessment the Preponderant Diagnostic Performances of Oligometastatic Prostate Cancer Using DCE-MRI of Tofts Model1The First Affiliated Hospital of Dalian Medical University, Dalian, China
Synopsis
It remains a challenge to diagnose the oligometastatic prostate cancer (PCa) due to the ambiguous definition of oligometastatic PCa. Previous studies had shown that dynamic contrast enhanced (DCE) - MRI imaging could be used to assess tumor aggressiveness. This study indicated that transfer constant (Ktrans) had the potential to assess the aggressiveness of PCa. And Ktrans combined with clinical characteristics (such as age and prostate specific antigen) had the higher diagnostic efficiency for oligometastatic and widely metastasis PCa.
Introduction
“Oligometastasis” was first proposed by Hellman and Weichselbaum in 1995 [1]. Oligomeric prostate cancer (PCa) indicates the intermediate state between local and advanced metastatic disease. In previous studies, the definition of oligometastatic PCa depended on the number of bone metastatic lesions (usually 3 to 5) [2]. Although the definition of oligometastatic PCa is not clear, studies have suggested that treatment of the primary tumor might provide a survival benefit to oligometastatic PCa patients [3-4]. Dynamic contrast enhanced (DCE) - MRI imaging could be used to assess tumor aggressiveness [5].Purpose
To evaluate the diagnostic performances of Tofts model (TM) in assessing oligometastatic PCa.Methods
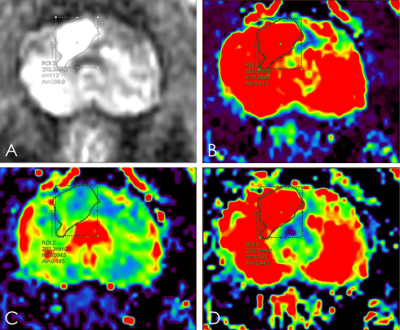
All PCa patients from January 2018 to December 2019, who underwent 3.0T MR scanning with DCE sequence were enrolled in this study. Patients with 1) treatment by chemotherapy, radiation therapy, endocrine therapy; 2) low image quality were excluded. Finally, 54 PCa patients were included in this study. Age ranged from 60 to 87 years, mean age 74±7.4 years. A 3.0T MR scanner (Signa HDxt, General Electric) and an 8-channel phased-array surface coil were used. DCE-MRI was performed using an axial T1-weighted spoiled gradient recalled sequence (repetition time 3 ms, echo time 1 ms, field of view 40×32 cm2, number of excitations 0.69, slice thickness 3.6 mm, slice spacing 0 mm, scan duration 3 minutes 42 seconds). Two T1-weighted images were acquired before a bolus of 0.1 mmol/kg Gd-DTPA was administered. A total of 40 time phases are scanned, each time phase is about 6s.GenIQ software was used to calculate statistics of the regions of interest (ROIs) of pharmacokinetic model parameters transfer constant (Ktrans), rate constant (Kep), and and extravascular extracellular space (Ve) (Figure 1). The region of interest (ROI) was placed on the largest slice of the tumor, and contained the whole tumor as much as possible. According to the number of bone metastatic lesions, the PCa patients were individed to three group: localized lesions group (no bone metastatic lesion), oligometastatic metastatic group (the number of bone metastatic lesions less than but equal to three) and widely metastatic group ( the number of bone metastatic lesions more than three). The intraclass correlation coefficient (ICC) was used to test the consistency of the two observers. The differences among groups were analyzed by Kruskal-Wallis test or One-way ANOVA test. Logistic regression was used to assess the quantitive metrics associated with the diagnosis of discriminating oligometastatic from widely metastasis PCa. Receiver-operating characteristic (ROC) curves to distinguish oligometastatic from widely metastasis PCa were estimated for quantitive metrics of DCE. The diagnostic potential was determined by calculating the area under the curve (AUC). Youden Index (Youden index = sensitivity + specificity - 1) was calculated and used for determining threshold values of quantitive metrics of DCE.Results
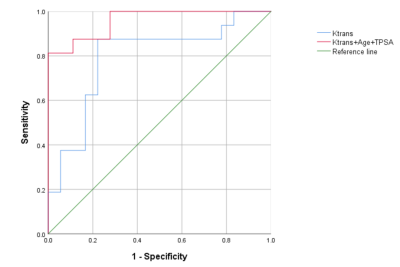
Inter-observer repeatability agreement was excellent in the orbital mass for all the TM parameters (ICC = 97%, 99% and 98% for Ve, Kep and Ktrans, respectively). There was a difference between oligometastatic group and widely metastasis group (P=0.034). There were no significant differences in Ve and Kep among the groups (P>0.05) . Logistic regression results showed that Ktrans can discriminate oligometastatic from widely metastasis PCa (OR<0.001,95% CI:0.000-0.028). The combination of Ktrans, age and PSA had the higher diagnostic potential for oligometastatic PCa (AUC, 0.958), compared with only Ktrans (AUC, 0.770) (Table 1, Figure 2).Discussion
Ktrans may be more efficient than Kep and Ve in the diagnosis of discriminating oligometastatic from widely metastasis PCa. The reason may be that the hypoxic microenvironment in the tumor causes the up-regulation of vascular endothelial growth factor (VEGF) with the tumor progressing . VEGF induces vascular leakage and increases interstitial fluid pressure (IFP). Importantly, Ktrans is related to microcirculation perfusion and microvascular permeability, which is why it can sensitively respond to the increase of IFP.Conclusion
Ktrans had the potential to assess the aggressiveness of PCa. Ktrans combined with clinical characteristics (such as age and PSA) had the higher diagnostic efficiency for oligometastatic and widely metastasis PCa.Acknowledgements
No acknowledgement found.References
[1] Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995 Jan;13(1):8-10. doi: 10.1200/JCO.1995.13.1.8. PMID: 7799047.
[2] Rii J, Sakamoto S, Yamada Y, Takeshita N, Yamamoto S, Sazuka T, Imamura Y, Nakamura K, Komiya A, Komaru A, Fukasawa S, Nakatsu H, Akakura K, Ichikawa T. Prognostic factors influencing overall survival in de novo oligometastatic prostate cancer patients. Prostate. 2020 Aug;80(11):850-858. doi: 10.1002/pros.24016. Epub 2020 Jun 5. PMID: 32501559.
[3] Rusthoven CG, Carlson JA, Waxweiler TV, Raben D, Dewitt PE, Crawford ED, Maroni PD, Kavanagh BD. The impact of definitive local therapy for lymph node-positive prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2014 Apr 1;88(5):1064-73. doi: 10.1016/j.ijrobp.2014.01.008. PMID: 24661660.
[4] Lin CC, Gray PJ, Jemal A, Efstathiou JA. Androgen deprivation with or without radiation therapy for clinically node-positive prostate cancer. J Natl Cancer Inst. 2015 May 9;107(7):djv119. doi: 10.1093/jnci/djv119. PMID: 25957435.
[5] Vos EK, Litjens GJ, Kobus T, Hambrock T, Hulsbergen-van de Kaa CA, Barentsz JO, Huisman HJ, Scheenen TW. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3 T. Eur Urol. 2013 Sep;64(3):448-55. doi: 10.1016/j.eururo.2013.05.045. Epub 2013 May 31. PMID: 23751135.
Figures