0933
Tumour vascular response to the FGFR inhibitor derazantinib assessed using susceptibility-contrast MRI with ferumoxytol1The Institute of Cancer Research, Sutton, United Kingdom, 2Basilea Pharmaceutica International Ltd, Basel 4005, Switzerland
Synopsis
Derazantinib (DZB), a FGFR inhibitor, has displayed potential anti-angiogenic effects in biochemical assays. In this study, we used in vitro and in vivo assays to explore this activity. Proliferation of human umbilical vein endothelial cells, their pVEGFR expression and downstream signalling, and vascular permeability in mouse skin are dose-dependently supressed by DZB. Susceptibility-contrast MRI using ferumoxytol demonstrated a reduction in fractional blood volume in subcutaneous colorectal cancer xenografts treated with DZB for 48h. This anti-angiogenic effect may be a relevant component of the activity of DZB in tumours bearing FGFR aberrations and may facilitate clinical activity against other solid tumours.
Introduction
Angiogenesis is important for the growth and development of tumours, and anti-angiogenic therapy has proved a successful treatment in the clinic for a number of solid tumours.Derazantinib (DZB) is an inhibitor of fibroblast growth factor receptors 1-3 (FGFR1-3), which has shown significant activity in phase 2 clinical trials in FGFR2-fusion positive intrahepatic cholangiocarcinoma.1 Biochemical kinase assays indicate potential activity against other important targets in oncology, including CSF1R and VEGFR2,2,3 and DZB can inhibit phosphorylation of CSF1R upon ligand stimulation in mouse macrophages ex vivo (GI50=100nM) at tolerable doses.2 To explore the potential of VEGFR2 as a target of DZB, in vitro and in vivo assays, including susceptibility-contrast MRI, were utilised, and comparisons made to the specific VEGFR inhibitor vatalanib.
Methods
Human umbilical vein endothelial cells (HUVECs) were cultured to 50% confluence and incubated with DZB (7nM-2µM) for 72h prior to the assessment of cell growth inhibition using a crystal violet assay to determine the GI50. Cells were also starved for 2h and treated for 1h with DZB (100nM or 1µM) or vatalanib (100nM or 1µM) followed by incubation with/out VEGF (100ng/ml) for 10min. Cells were then harvested in protein lysis buffer, and the lysates assessed by Western blot using specific antibodies against phospho-VEGFR2 and phospho-MEK1/2 (Cell Signaling Technology). Western blot images were quantified using the EvolutionCapt programme.To assess the effect of DZB on vascular permeability, male ICR mice were treated for 24-72h with DZB (25-100mg/kg p.o. daily), vehicle (dimethyl acetamide:cremophor EL:propylene glycol:acetate buffer 0.2M pH5, 1:1:3:5) or vatalanib as a positive control (50% PEG300 in PBS, 50mg/kg, p.o. twice a day).4 A Miles assay was then performed, measuring leakage of Evans blue into the skin 3h after i.v. injection (40mg/kg) and 0.5h after i.d. injection of VEGF (20ng/0.1ml).
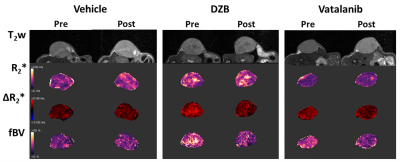
For MRI studies, human SW1222 colorectal cancer cells were grown subcutaneously on the flank of female athymic nude mice and imaged when tumours reached 250±50mm3. Susceptibility-contrast (SC) MRI was performed on a Bruker Biospec 7T system over a 3cm field of view using a 4cm volume coil with the tumour surrounded by dental paste to minimise susceptibility artefacts. Multi-slice T2-weighted images (RARE; TR=4500ms, TEeff=36ms) were acquired for planning and tumour volume calculation. Multi-gradient recalled echo (MGRE) images (TR=200ms, 8 echoes 3-24ms) were acquired from 3 central 1mm thick transverse slices to quantify R2* prior to and following intravenous administration of USPIO particles (150µmolFe/kg, ferumoxytol). MGRE data were fitted on a voxel-by-voxel basis using a Bayesian maximum a posteriori approach, providing maps of R2*, ΔR2*USPIO and fractional blood volume (fBV); median values for whole tumour ROIs are reported.5
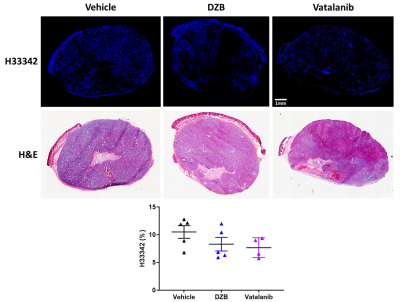
Mice were treated for 48h with DZB (80mg/kg daily, n=6), vehicle (n=5) or vatalanib (50mg/kg twice a day, n=4), following which the SC-MRI protocol was repeated. The perfusion marker Hoechst 33342 (15mg/kg) was given i.v. for 1 minute before the tumour was excised and snap frozen. Perfused vasculature was assessed on 10µm sections by fluorescence microscopy and sections were then stained with H&E.
Results
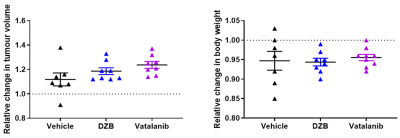
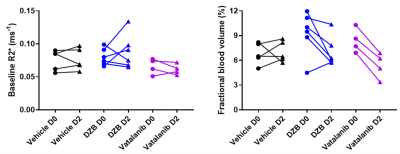
HUVEC proliferation was dose dependently inhibited by DZB with an approximate GI50 of 400nM (Figure 1a). DZB also inhibited expression of pVEGFR2 and downstream pMEK1/2 in a similarly dose dependent manner (Figure 1b). Miles assays of vascular permeability in the skin were more sensitive to the effects of DZB and vatalanib at 24h than at 48 or 72h. At 24h, DZB dose dependently decreased Evans blue permeability with 75mg/kg resulting in a mean reduction of 58% compared to vehicle treated controls; in comparison, vatalanib caused an 88% reduction (p<0.001; one-way ANOVA) (Figure 1c).Neither DZB nor vatalanib affected the growth rate of SW1222 tumours over 48h treatment, and the doses given were well tolerated (Figure 2). Figure 3 shows example parametric maps of baseline R2*, ΔR2*USPIO and fBV prior to and following treatment with DZB, vatalanib or vehicle. No change in baseline R2* over 48h treatment was observed in any of the treatment groups (Figure 4). USPIO-induced ΔR2* and fBV were reduced by an average of 21% and 20%, respectively, in DZB treated tumours (Figure 4). Significant decreases in ΔR2*USPIO and fBV of 47% and 37%, respectively, were observed in vatalanib treated tumours. No change in ΔR2*USPIO or fBV was observed in vehicle treated tumours.
Fluorescence microscopy of Hoechst 33342 demonstrated that both agents tended to reduce the functional perfused vasculature but this was not significant in this cohort (Figure 5).
Discussion
DZB inhibits HUVEC proliferation and VEGFR2 signalling in vitro at concentrations achievable in mice and patients, and decreases skin permeability in vivo.Absolute values of tumour fBV determined using SC-MRI with ferumoxytol were in agreement with those previously reported in SW1222 xenografts and other subcutaneous tumour models using alternative USPIO particle preparations.5,6
DZB decreased USPIO-induced ΔR2* and fBV in SW1222 xenografts in a weaker, but comparable, manner to the specific VEGFR-inhibitor vatalanib. The data are consistent with a moderate anti-angiogenic effect, which may be a relevant component of the activity of DZB in solid tumours bearing FGFR aberrations and could facilitate clinical activity against solid tumours without FGFR modifications.
Acknowledgements
We acknowledge CR-UK programme grant (C16412/A27725).References
1. Mazzaferro V, El-Rayes BF, Droz Dit Busset M, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120:165-171.
2. McSheehy P, Bachmann F, Forster-Gross N, et al. Derazantinib (DZB): A dual FGFR/CSF1R-inhibitor active in PDX-models of urothelial cancer. Mol Cancer Ther. 2019;18:LB-C12.
3. Hall TG, Yu Y, Eathiraj S, et al. Preclinical Activity of ARQ 087, a Novel Inhibitor Targeting FGFR Dysregulation. PLoS One. 2016;11:e0162594.
4. Wood JM, Bold G, Buchdunger E, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178-2189.
5. Robinson SP, Boult JKR, Vasudev NS, Reynolds AR. Monitoring the vascular response and resistance to sunitinib in renal cell carcinoma in vivo with susceptibility contrast MRI. Cancer Res. 2017;77:4127-4134.
6. Burrell JS, Bradley RS, Walker-Samuel S, et al. MRI measurements of vessel calibre in tumour xenografts: comparison with vascular corrosion casting. Microvasc Res. 2012;84:323-329.
Figures