0930
MR elastography reveals a marked increase in breast cancer viscoelasticity in vivo following hyaluronan degradation by PEGPH201Radiotherapy & Imaging, Institute of Cancer Research, London, United Kingdom, 2Institutes of Brain Science, Fudan University, Shanghai, China, 3Royal Marsden NHS Foundation Trust, Sutton, United Kingdom, 4Halozyme Therapeutics, San Diego, CA, United States, 5Division of Imaging Sciences and Biomedical Engineering, King's Health Partners, St Thomas's Hospital, London, United Kingdom
Synopsis
We hypothesised that hyaluronan (HA) degradation by PEGPH20 may alter tumour viscoelasticity measured by MR elastography (MRE). MRE was performed before and after PEGPH20 in three orthotopic breast tumour models (4T1, 4T1/HAS3 and MDA-MB-231 LM2-4). Viscoelastic properties did not change following PEGPH20 in 4T1 and 4T1/HAS3 tumours. However, a dramatic PEGPH20-induced increase in tumour viscoelasticity was seen in MDA-MB-231 LM2-4 tumours, likely the result of collagen network rearrangement and not HA degradation alone. Although MRE is unlikely to provide a robust biomarker of PEGPH20 response, these data clearly demonstrate that MRE-derived biomarkers can inform on increased tumour stiffness in vivo.
Introduction
Breast cancer is often characterised by a dense stroma, with accumulation of extracellular matrix (ECM) components including hyaluronan (HA) and collagen. Increased stiffness is associated with inefficient drug delivery, tumour progression and metastasis. PEGPH20 is a PEGylated, recombinant, human hyaluronidase which can enzymatically degrade HA. PEGPH20 has been shown pre-clinically to improve drug delivery and treatment response through reduction of tumour interstitial fluid pressure and decompression of blood vessels[1-3].The development of ECM-targeted therapies such as PEGPH20 may be accelerated by MRI biomarkers which inform on therapeutic efficacy[4-7]. MR elastography (MRE) can non-invasively map and quantify tumour viscoelastic properties in vivo, and inform on breast tumour response to collagen degradation[8]. HA degradation by PEGPH20 is associated with a reduction in tumour water content and collapse of the extracellular space[2, 3]. These biomechanical responses may impact tumour viscoelasticity, providing a strong rationale to exploit MRE to assess tumour response to PEGPH20.
Methods
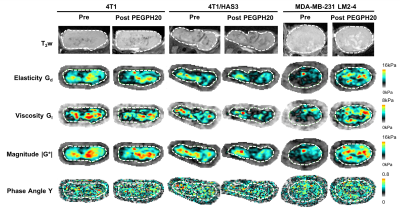
Experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1996. Murine 4T1 (n=12) and HA synthase 3 overexpressing 4T1 (4T1/HAS3; n=12) breast tumours were propagated orthotopically in female BALB/c mice. Human luc-MDA-MB-231 LM2-4 breast tumours (n=12) were propagated orthotopically in female athymic NCr-Foxn1nu mice. 4T1 (453 ± 23 mm3), 4T1/HAS3 (362 ± 33 mm3), and MDA-MB-231 LM2-4 (456 ± 38 mm3) tumours were imaged before and 24 hours after treatment with either saline or 1 mg/kg PEGPH20.Anatomical T2-weighted MRI and MRE (TE = 30 ms, TR = 504 ms, vibration frequency = 1000 Hz) were performed on a Bruker 7T horizontal MRI system[9]. Parametric maps of elasticity (Gd), viscosity (Gl), the magnitude of the complex shear modulus (|G*| = √[Gd2+Gl2]) and the normalised phase angle (Y = 2/π[tan-1[Gl/Gd]])[10] were reconstructed isotropically from three 0.3 mm thick axial slices over a 19.2x19.2 mm FOV. At the extremes, tissue is pure elastic (Y = 0) or pure viscous (Y = 1)[10]. Median values for each tumour were calculated from a region of interest (ROI) which covered viable tumour tissue and excluded necrosis.
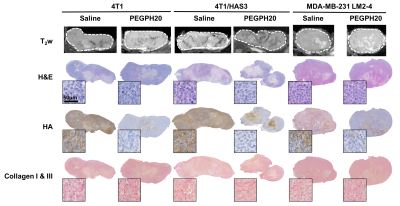
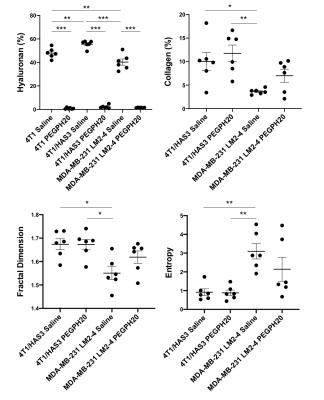
MRI-aligned FFPE tissue sections (5 μm) were stained with H&E, HTI-601[11] (HA) and picrosirius red (collagen I & III). Percent HA was quantified using Definiens Tissue Studio®. Percent collagen, fractal dimension and entropy were quantified as described previously[8]. Fractal dimension and entropy describe 2D collagen distribution[12].
Statistical analyses (Student’s unpaired t-test or one-way ANOVA) were performed using Prism 8 (GraphPad) with a significance level of 5%. Data are presented as mean ± SEM.
Results
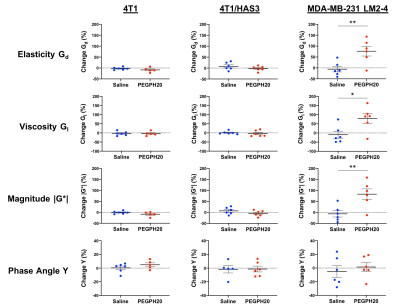
Pre-treatment values of Gd, Gl and |G*| were highest in 4T1 tumours, followed by 4T1/HAS3 tumours, and lowest in MDA-MB-231 LM2-4 tumours (Figure 1). Y was similar across all tumour models. Whilst there was no change in Gd, Gl and |G*| following PEGPH20 in the 4T1 and 4T1/HAS3 tumours, a large and significant increase in Gd (77 ± 22 %), Gl (79 ± 26 %) and |G*| (83 ± 24 %) was determined in MDA-MB-231 LM2-4 tumours in response to PEGPH20 (Figures 1 & 2). No PEGPH20-induced change in Y was apparent.HA accumulation was highest in 4T1/HAS3 tumours, followed by 4T1 tumours and lowest in MDA-MB-231 LM2-4 tumours. PEGPH20 effectively degraded HA in all tumour models (Figures 3 & 4). 4T1/HAS3 tumours had more collagen, with higher fractal dimension and lower entropy compared to MDA-MB-231 LM2-4 tumours. A trend suggests PEGPH20 increased collagen and fractal dimension, and decreased entropy, in the MDA-MB-231 LM2-4 tumours.
Discussion
MRE revealed that 4T1 tumours had higher Gd, Gl and |G*| compared to 4T1/HAS3 tumours, suggesting HA accumulation contributes to lower viscoelasticity. Despite this, HA degradation by PEGPH20 induced no change in viscoelastic properties of 4T1 and 4T1/HAS3 tumours. These data suggest that HA accumulation during tumour development is associated with a softer and less viscous phenotype, but the extent of HA itself does not significantly contribute to tumour viscoelasticity measured by MRE.PEGPH20 induced an unprecedented ~80% increase in the viscoelastic properties of MDA-MB-231 LM2-4 tumours. Given the 4T1 and 4T1/HAS3 data, this response is presumably not due to HA degradation alone, and is more likely a consequence of other biomechanical-related changes. Collagen is a determinant of tumour viscoelasticity as measured by MRE[8]. Quantitative histology suggests a higher collagen content, coupled with increased collagen complexity (fractal dimension) and a more homogeneous collagen distribution (lower entropy) in the PEGPH20 treated MDA-MB-231 LM2-4 tumours. This is consistent with a more organised collagen network and may explain the marked increase in tumour stiffness following PEGPH20.
Conclusion
In conclusion, HA accumulation is associated with lower viscoelastic properties, although this is likely to be dependent on how HA is interacting with its surrounding microenvironment. PEGPH20 had different, model-specific, effects on tumour viscoelasticity. Therefore, it is unlikely MRE will provide a robust biomarker of HA degradation.The dramatic PEGPH20-induced increase in stiffness measured in MDA-MB-231 LM2-4 tumours is likely a consequence of collagen network rearrangement. Many oncological MRE studies report tumour softening associated with cell death following treatment[9, 10]. The data herein clearly demonstrate the sensitivity of MRE to inform on increased tumour stiffness, and support further evaluation of Gd, Gl and |G*| as binary imaging biomarkers of tumour treatment response.
Acknowledgements
We acknowledge support from the Cancer Research UK Centre at the ICR, European Union Horizon 2020 Research and Innovation Programme (Grant #668039), the Rosetrees Trust (M593), Cancer Research UK support to the Cancer Imaging Centre at the ICR, in association with the MRC and Department of Health (England) (C1060/A16464), Cancer Research UK grant C16412/A27725 and a Children with Cancer UK Research Fellowship.References
1. Provenzano, P.P., et al., Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 2012. 21(3): p. 418-29.
2. Thompson, C.B., et al., Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther, 2010. 9(11): p. 3052-64.
3. DuFort, Christopher C., et al., Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophysical Journal, 2016. 110(9): p. 2106-2119.
4. Hingorani, S.R., et al., Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res, 2016. 22(12): p. 2848-54.
5. Reeves, E.L., et al. 0143 Breast tumour response to PEGPH20-induced stromal modulation assessed by multiparametric MRI. in Proc. Intl. Soc. Mag. Reson. Med. 28. 2020.
6. Reeves, E.L., et al. 4850 Intrinsic susceptibility MRI can inform on tumour vascular decompression in response to the stromal targeted therapy PEGPH20. in Proc. Intl. Soc. Mag. Reson. Med. 28. 2020.
7. Cao, J., et al., Dynamic contrast-enhanced MRI detects responses to stroma-directed therapy in mouse models of pancreatic ductal adenocarcinoma. Clin Cancer Res, 2018.
8. Li, J., et al., Investigating the Contribution of Collagen to the Tumor Biomechanical Phenotype with Noninvasive Magnetic Resonance Elastography. Cancer Research, 2019.
9. Li, J., et al., Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. British Journal of Cancer, 2014. 110(7): p. 1727-1732.
10. Schregel, K., et al., Magnetic Resonance Elastography reveals effects of anti-angiogenic glioblastoma treatment on tumor stiffness and captures progression in an orthotopic mouse model. Cancer Imaging, 2020. 20(1): p. 35.
11. Jadin, L., et al., Characterization of a Novel Recombinant Hyaluronan Binding Protein for Tissue Hyaluronan Detection. Journal of Histochemistry & Cytochemistry, 2014. 62(9): p. 672-683.
12. Nieskoski, M.D., et al., Collagen Complexity Spatially Defines Microregions of Total Tissue Pressure in Pancreatic Cancer. Scientific Reports, 2017. 7.
Figures