0929
31P MRSI in tumor-bearing mice at 9.4T1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Center for Preclinical Research, Core Facility Tumor Models, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Division of Molecular Embryology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
31P MRSI allows for the non-invasive investigation of energy metabolism in vivo and is therewith of interest for research on novel therapies for cancer. The purpose of this study was to investigate suitable acquisition strategies for 31P MRSI in tumor-bearing mice at B0=9.4T in order to monitor in future studies effects of novel therapies affecting energy metabolism and pH values. 31P MRSI datasets with a spatial resolution of (2.5x2.5x7.5) mm³ obtained in 50 minutes enabled the quantification of signals in diseased tissue, while maintaining acceptable separation to healthy tissue.
Introduction
Phosphorus magnetic resonance spectroscopic imaging (31P MRSI) is a non-invasive method for the investigation of energy metabolism in vivo. It enables the localized detection of 31P metabolite signals, as well as mapping of the intracellular pH value1,2. Therewith, 31P MRSI is of interest for research on novel therapies for cancer.Earlier studies using 31P MRS in animal studies using surface coils mainly focused on acquiring unlocalized spectra3 or applied single voxel methods4,5, which only allows the investigation of a small region of the measured volume. However, larger spatial coverage may be desirable in order to compare diseased tissue to healthy control tissue within the same measurement.
The purpose of this study was to investigate suitable acquisition strategies for 31P MRSI in tumor-bearing mice at B0=9.4T in order to monitor in future studies effects of novel therapies affecting energy metabolism and pH values.
Methods
Nude mice with DLD1 xenografts implanted subcutaneously on the flank were examined on a 9.4T small animal MR scanner (Bruker) using a double-resonant 31P-1H volume resonator (diameter 40mm). Various 2D and 3D protocols with different matrix sizes (from (10x10) to (14x14) points in-plane), spatial resolutions (from 3 to 6.75mm in-plane) and acquisition durations (from 27 to 58min) were tested to assess detectability limits.Mice examinations were approved by the local regulatory authorities under G284-15.Two exemplary protocols are presented in the following. In both protocols, 31P MRSI data were acquired using an acquisition-weighted 2D CSI sequence with a slice thickness of 7.5mm, one with an in-plane spatial resolution of (3×3)mm² (Ttot=46min), and the other with (2.5x2.5)mm² (Ttot=50min). During 25% of the readout time, a 1H decoupling train (Mlev16) was applied to enhance 31P signals.
Both MRSI datasets were processed by one-fold spatial zerofilling and application of a Gaussian filter in the time domain. Two regions of interest (ROIs) covering muscle tissue and the marginal part of the tumor were defined on an overlay of the fitted PCr amplitude with an 1H image using MITK6 (figures 1,3). 31P spectra from the ROIs were summed up after correcting zero-order phases and B0-offsets, and evaluated using a home-built Matlab (The MathWorks) implementation of the AMARES algorithm7. Intracellular pH value was calculated using the chemical shift difference between inorganic phosphate (Pi) and Phosphocreatine (PCr) according to the modified Henderson-Hasselbalch equation1.
Results
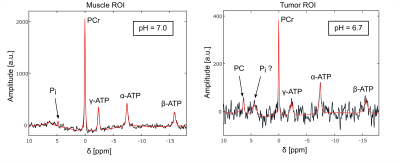
With the presented protocols, the obtained localized 31P MR spectra were of good quality where B0 homogeneity was sufficient (figures 2 and 4). In the region of the back muscle, spectral quantification of all expected resonances (i.e. PCr, ATP, Pi) could be achieved reliably, enabling the calculation of an intracellular pH value of around 7.0 in the first dataset, and of around 7.1 in the second.The spectra from the tumor ROIs show a significantly lower intensity for the PCr and ATP resonances. In the first dataset, a sharp resonance from PC was resolved at δ=6.3ppm (figure 2, right). Assuming the broad peak at δ=4.4ppm resulted from compartmentalized contributions of Pi, a pH value of around 6.7 can be determined in the tumor ROI.
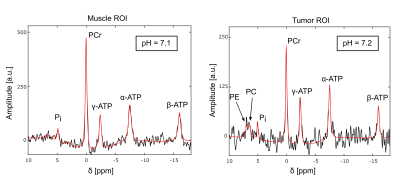
In the tumor ROI of the second dataset (figure 4, right), broad PE and PC resonances were resolved, and a sharp Pi resonance at δ=5ppm. Therewith a pH of around 7.2 was determined.
In the tested 3D MRSI protocols, the spectral quality was poorer compared to the 2D acquisitions, due to the more inhomogeneous B0 obtained after shimming. In the other tested 2D MRSI protocols, similar spectral quality could be obtained as demonstrated in figures 2 and 4, as long as the B0 homogeneity was comparable.
Discussion
In comparison to earlier studies using surface coils3-5, the use of a volume resonator with a diameter of 40mm in order to ensure a larger spatial coverage posed a significant challenge concerning sufficient SNR in this study. In addition, due to the position of the tumor in the upper flank relatively close to the abdomen, B0 shimming was challenging and only sufficient in a limited region of the measured volume. Therefore, the tested 3D CSI protocols with low resolution ((5-6.75)mm in-plane) did not yield spectra of sufficient quality. However, 2D protocols with higher resolution ((2.5–3.5)mm in-plane) yielded localized 31P spectra of good quality in a reasonable measurement time (30-50min), in the region where B0 homogeneity was sufficient.The therewith-acquired 31P spectra from the back muscle are in line with expectations (i.e. clear resonances of PCr, ATP and Pi, pH around 7).
However, spectra from tumor ROIs had not in all cases pure signatures from tumor tissue (e.g. increased Phosphomonoester and -diester signals, higher pH value). This is due to signal contamination from the high signal from adjacent muscle tissue. The broad signal around δ=4.4ppm in the first dataset (figure 2, right) might result from compartmentalized contributions of Pi, which would be in line with the finding of partial necrosis within the tumor, which was verified in a subsequent resection of the tumor.
Conclusion
31P MRSI with large spatial coverage in mice at B0=9.4T is feasible, despite several challenges. Datasets acquired with a spatial resolution of (2.5x2.5x7.5)mm³ in 50 minutes enabled the quantification of signals in diseased tissue, while maintaining acceptable separation to healthy tissue.Acknowledgements
No acknowledgement found.References
1. de Graaf RA. In Vivo NMR Spectroscopy: Principles and Techniques: 2nd Edition.; 2007.
2. Korzowski, A, Weinfurtner, N, Mueller, S, et al. Volumetric mapping of intra‐ and extracellular pH in the human brain using 31P MRSI at 7T. Magn Reson Med. 2020; 84: 1707– 1723.
3. Zhou R, Bansal N, Leeper DB, Glickson JD. Intracellular acidification of human melanoma xenografts by the respiratory inhibitor m-iodobenzylguanidine plus hyperglycemia: a 31P magnetic resonance spectroscopy study. Cancer Res. 2000 Jul 1;60(13):3532-6. PMID: 10910065.
4. Rackayova, V., Braissant, O., McLin, V.A. et al. 1H and 31P magnetic resonance spectroscopy in a rat model of chronic hepatic encephalopathy: in vivo longitudinal measurements of brain energy metabolism. Metab Brain Dis 31, 1303–1314 (2016).
5. Bakermans, AJ, Abdurrachim, D, van Nierop, BJ, Koeman, A, van der Kroon, I, Baartscheer, A, Schumacher, CA, Strijkers, GJ, Houten, SM, Zuurbier, CJ, Nicolay, K, and Prompers, JJ (2015), In vivo mouse myocardial 31P MRS using three‐dimensional image‐selected in vivo spectroscopy (3D ISIS): technical considerations and biochemical validations. NMR Biomed., 28, 1218– 1227.
6. Nolden M, Zelzer S, Seitel A, et al. The medical imaging interaction toolkit: Challenges and advances: 10 years of open-source development. Int J Comput Assist Radiol Surg. 2013;8(4):607-620.
7. Vanhamme L, Van Huffel S. AMARES: Advanced Method for Accurate, Robust and Efficient Spectral fitting of MRS data with use of prior knowledge. J Magn Reson. 1997;43(129):1-2.
Figures
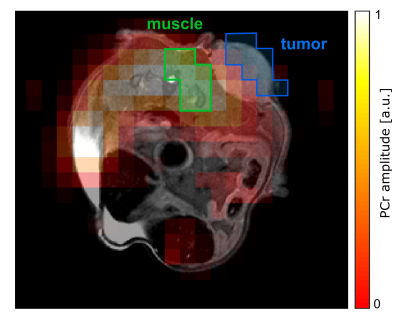
Figure 1: Transversal map of the fitted PCr amplitude overlaid on the morphological 1H image in the slice covering the tumor. Data was acquired with protocol 1: matrix size=10x10, FOV=(30x30)mm², Hamming-weighted k-space averaging with 364 central averages, postprocessing with zerofilling-factor 2. The tumor ROI (blue) was drawn on the marginal part of the tumor to reduce signal contamination from muscle. The muscle ROI (green) includes the same number of voxels as the tumor ROI.