0926
In-Vivo Cell Tracking of Murine Natural Killer Cells in Lymphoma by Fluorine-19 MRI1Medical Physics, University of Wisconsin, Madison, Madison, WI, United States, 2Radiology, University of Wisconsin, Madison, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin, Madison, Madison, WI, United States, 4Carbone Cancer Center, University of Wisconsin, Madison, Madison, WI, United States, 5Pediatrics, University of Wisconsin, Madison, Madison, WI, United States
Synopsis
Fluorine-19 (19F) MRI can monitor various cell types in vivo for days to weeks after injection of 19F-labeled cells. To establish cell viability Green Fluorescent Protein (GFP+) murine natural killer (NK) cells were labeled ex vivo using red-fluorescent perfluoropolyether (PFPE-red) and delivered intratumorally into 3 lymphoma-bearing mice. Flow cytometry validated that the infused NK cells retain their 19F label out to 6 days post-injection (PI). Cells were then tracked and quantified via 1H/19F MRI out to 6 days PI. Quantification of the infused cells indicate 87% and 70% were detectible at days 0 and 6, respectively.
Background and Purpose
Currently, our understanding of the biodistribution, dynamics, and persistence of adoptively transferred cells used in a cancer immunotherapy setting, is lacking.1,2 In various studies, Fluorine-19 (19F)-MRI has demonstrated the ability to track and quantify adoptively transferred immune and stem cells in vivo for days to weeks post-injection (PI). For many cell types, such as NK cells, it is unknown what fraction of the cells retain the 19F label and for what duration PI. Important biological questions include: Are the infused cells still viable? Is the label still colocalized within the original cell population? Is any off-labeling present within the imaging region? Using GFP+ NK cells and PFPE-red, identification of green and red fluorescence can be leveraged to confirm labeled NK cells are still viable and retain the 19F label. The purpose of this study was, therefore, (1) to track and quantify an intratumorally injected PFPE-red (fluorescently active 19F MRI probe3) labeled GFP+ NK cell population within 3 lymphoma-bearing mice out to 6 days PI and (2) validate the viability of the injected cells and evaluate label retention at the conclusion of day 6, via flow cytometry (FC) of the dual-fluorescent cells.Methods
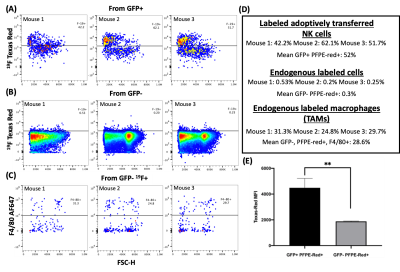
GFP+ NK cells from C57BL/6 mice were isolated and incubated for 24-hours with a red-fluorescent PFPE nanoemulsion (PFPE DM Red; Celsense, Pittsburgh, PA). Cell labeling density was then determined by acquiring 19F NMR spectra of lysed-NK cells that were labeled with various PFPE-red concentrations. Spectra were acquired via a 9.4T NMR spectrometer (Agilent Technologies, Santa Clara CA) with a TR of 9.0 s, 90-degree flip angle, 14.075 kHz spectral width, and 64 averages. Trifluoroacetic Acid was also added to the cell lysate sample for use as a 19F chemical shift and quantification standard. Guided from the NMR results, GFP+ NK cells were then labeled with 4 mg/mL of PFPE-red, washed, counted, and injected in aliquots of 5.3⨯105 cells/50 μL, intratumorally to three tumor-bearing mice. Two weeks prior to injection, mice were injected subcutaneously in the flank with 10x106 EL4 T cell lymphoma cells. Once the tumors reached 5 mm in diameter, PFPE-red labeled GFP+ NK cell infusions were given. In vivo MRI data was obtained at days 0, 3, and 6 PI via a 4.7T small animal MRI (Agilent Technologies, Santa Clara CA) using a custom-built 1H/19F quadrature coil. Anatomic images were acquired with a T2-weighted fast spin echo (FSE). 19F images were acquired using a FSE (TR/TE = 1000/20.0 ms, 1.1⨯1.1⨯2.0 mm3 voxel size, 12 echoes, 400 averages, ~25-minutes) where a chemical-saturation pulse was added to remove isoflurane signal contamination. 1H and 19F images were overlaid using MATLAB 2019A (Mathworks, Natick, MA) to produce composite images. In vivo quantification from the 1H/19F images was achieved via region-of-interest (ROI) analysis (adapted from previous studies4,5) of the tumor and a PFPE quantification reference vial that was placed contralateral to the tumor. Images were thresholded at four times the standard deviation of the bias-corrected background noise to mitigate noise contributions. The total 19F signal in the tumor and the mean signal in the reference were then measured. At the end of day 6 PI, mice were sacrificed and the tumors were excised, stained with F4/80 antibody, and processed via Attune™ NxT Flow Cytometer (Thermo Fisher Scientific), with subsequent analysis performed with FlowJo™ software (BD, Ashland, OR). Cells were analyzed by forward scatter/side scatter to gate on live cells, and then gated as tumor cells (GFP-PFPE-red-), endogenous phagocytic cells (F4/80+GFP-PFPE-red+), or viable adoptively transferred NK cells (GFP+, PFPE-red+).Results
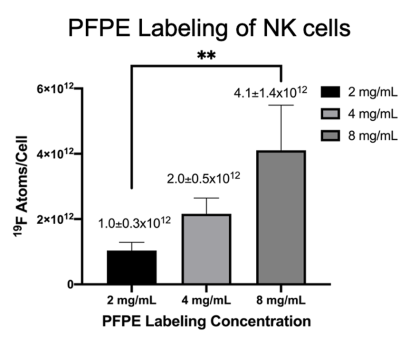
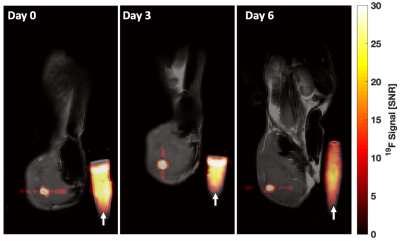
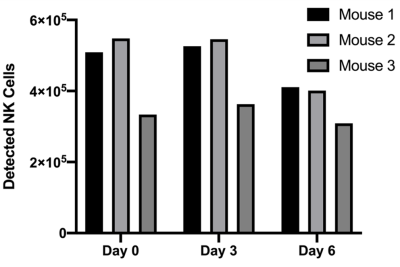
NMR analysis, (Figure 1), indicated 4 mg/mL produced the largest level of PFPE-red uptake of 2.0±0.5⨯1012 19F atoms/cell without severely effecting viability and function, which is approximately 6 times greater labeling than was achieved in our lab’s previous work with human-derived NK cells4. 1H/19F MRI detected signal within each mouse at all time points, as shown in a representative mouse in Figure 2. Cellular quantification, (Figure 3), detected 87%, 90%, and 71% of the initially infused NK cells. FC validated that the PFPE-red label persists within the majority of GFP+ NK cells at the end of day 6 (Figure 4); however, there appears to be a small amount of off-labeling within lymphoma cells and tumor-associated macrophages (TAMs).Conclusions
Adoptively transferred NK cells were successfully imaged and quantified in vivo out to 6 days post intratumoral injection into lymphoma. Postmortem FC of the tumors provides validation that the majority of adoptively transferred NK cells retain their PFPE-red label (GFP+, PFPE-red+) and are viable out to 6 days PI. A small amount of off-labeling was detected in the endogenous lymphoma cells (GFP-, PFPE-red+) and TAMs (GFP-, PFPE-red+, F4/80+); however, based upon FC, their signal contribution is significantly less than the infused cell population. This off-labeling may be due to PFPE-red label being released from the infused NK cells upon their death, that are later phagocytosed by the endogenous immune cells. This work demonstrates tracking of adoptively transferred NK cells within a syngeneic murine lymphoma model is feasible to at least 6 days PI, with validation of NK viability and 19F label retention via FC.Acknowledgements
This project was supported by the Radiological sciences training grant T32-CA009206 to Lawrence Lechuga, AOF/SciMed GRS Fellowship to Lawrence Lechuga, R01 CA215461-01A1 (Capitini, PI), P30 CA014520 Supplement to Cancer Center (Capitini, PI), Grant support for multi-nuclear spectroscopy development by GE Healthcare, Grant support from Celsense, Inc.
References
- Chapelin, F., Capitini, C. M. & Ahrens, E. T. Fluorine-19 MRI for detection and quantification of immune cell therapy for cancer. J. Immunother. Cancer 6, 1–11 (2018).
- Varani, Auletta, Signore & Galli. State of the Art of Natural Killer Cell Imaging: A Systematic Review. Cancers (Basel). 11, 967 (2019).
- Janjic, J. M., Srinivas, M., Kadayakkara, D. K. K. & Ahrens, E. T. Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. J. Am. Chem. Soc. 130, 2832–2841 (2008).
- Bouchlaka, M. N. et al. 19F-MRI for monitoring human NK cells in vivo. Oncoimmunology 5, 1–12 (2016).
- Srinivas, M., Morel, P. A., Ernst, L. A., Laidlaw, D. H. & Ahrens, E. T. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 58, 725–734 (2007).
Figures