0925
Deuterium metabolic imaging (DMI) of glucose highlights pancreatic cancers in two mice models1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2Center for Magnetic Resonance in Biology and Medicine, Marseille, France, 3The Moross Integrated Cancer Research Center, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Deuterium Metabolic Imaging (DMI) was used to follow metabolism in two pancreatic cancer mouse models, after tail-vein administration of 2H6,6’-glucose. Metabolic maps for the glucose and for its metabolic products 2H3,3’-lactate and 2H-water were measured over a time course of 2 h by 2H chemical shift imaging (CSI) at 15.2 T. Abdominal images exhibited sharp and specific lactate signals, which generated exclusively in the tumors. Thus, DMI may open valuable opportunities for non-invasively imaging pancreatic cancer –including its diagnosis and treatment.
INTRODUCTION
Recent studies have concluded that tracking and imaging the fate of deuterated metabolites, could map non-invasively glycolysis and other biochemical processes in animals and humans. A promising avenue of this deuterium metabolic imaging (DMI) approach involves looking at externally administered 2H6,6’-glucose, as it is taken up and metabolized in various organs into different products as a function of time.1-6 This study employs DMI to study two strains of orthotopic pancreatic cancer mice models (PAN-02 and KPC) at 15.2 T. For both cases the generation of a clear 2H3,3’-lactate signature localized solely in the tumors, evidenced the ability of this approach to non-invasively image this manifestation of the Warburg effect.METHODS
All experiments were preapproved by an institutional IACUC. Pancreatic cancer was investigated with two models: n=7 black mice were implanted with PAN-02 orthotopic tumors; n=5 were implanted with KPC cells.7 All experiments were recorded in a Bruker Biospec scanner at 15.2T running Paravision 6. A “sandwich” coil setup was used for scanning, whereby a 20x45 mm 1H butterfly surface coil (650 MHz) was placed underneath the sidewise-laying mouse, and a customized 20 mm single-loop surface coil tuned to 2H’s (99.8 MHz) was placed on top of the belly. Anatomical images were collected using 1H TurboRARE (28-38 slices, 0.5 mm slice thickness, 0.2 mm in-plane resolution). For DMI mice were catheterized in their tail-veins, and intravenously administered 99.5%-enriched 2H6,6’-glucose in PBS, at a dose of 3 g/kg as a single bolus 0.25 mL injection within 60 sec. Non-localized 2H NMR data sets were acquired with a ≈20° excitation pulses, 100 ms acquisition time, 0 ms recycle delay, 128 transients. Spatially-resolved 2H CSI data were collected using ≈90° flip-angles (≈ 5mm slice thickness), 60 ms acquisition times, 40x40 mm2 FOVs, 8x8 k-matrices (zero-filled to 32x32) sampled on a Cartesian grid, and repetition times TR = 95 ms. A k-dependent weighted-average, collecting 320 signal averages for k=0 and progressively less for its periphery, was used. This delivered a data set every ~8 min. Non-localized 2H NMR and slice-selective 2H CSI sets were acquired in alternated, interleaved blocks spaced ~15-20 minutes, starting from before and continuing for ca. 120 minutes after deuterated glucose injection. 2H spectra and images were reconstructed in Matlab® using custom written code.RESULTS
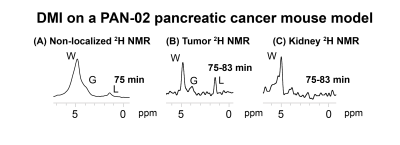
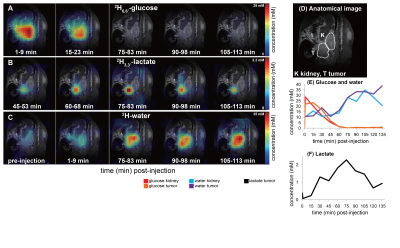
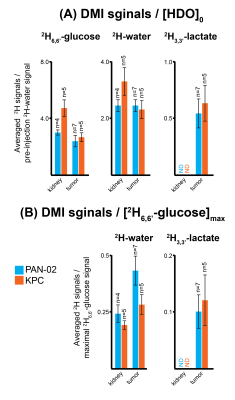
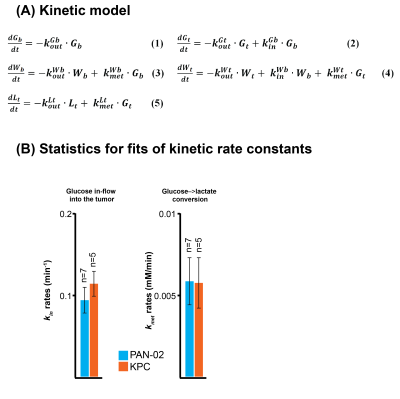
2H NMR and spatially-resolved 2H CSI data recorded after 2H6,6’-glucose injection, showed the formation of 2H3,3’-lactate and 2H-water for both pancreatic cancer models (Figure 1A). When implemented with localization, these experiments only revealed 2H3,3’-lactate in the tumor voxels (Figures 1B, 1C). Figures 2A-2C exemplify this with 2H CSI data, where images that were spectrally-resolved for glucose, lactate and water, are displayed for selected post-injection time points. Localization of 2H3,3’-lactate can be detected exclusively in the tumor discernable in the 1H anatomical image (Figure 2D); analogous behaviors were observed for other PAN-02- and KPC-implanted mice. Typical metabolic concentration changes as a function of time are presented in Figures 2E-2F, calculated on the basis of an initial natural-abundance HDO background signal corresponding to 10 mM. Glucose levels in the kidney and tumor increase with the initial bolus injection and then gradually decrease, whilst water is steadily generated over the course of two hours. 2H3,3’-lactate reaches lower concentrations, but always peaks ≈60-90 min after injection. Figure 3 gives two different views of the overall metabolic levels reached in these studies: Panel A shows them normalized by the pre-injection water signal; panel B normalized by the maximum glucose observed for each animal/injection. Voxels chosen for these analyses included the kidneys as a well-defined, healthy organ, and the tumorous tissue. The perfusion of the injected glucose is remarkably similar in both tissues. Also similar are the lactate levels detected in the tumors, for both pancreatic cancer strains. The experimental time traces were fit to a simple body/tumor two-compartments kinetic model based on the first-order differential equations shown in Figure 4A. Here G, W and L stand for the three metabolites, the b/t denote the compartments, and k are rates of a metabolite’s in-flow or out- from the compartment, or for its intra-compartmental metabolic generation from glucose. Fits of the experimental data provide two cancer-relevant rates: the flow of glucose into the tumor, and its rate to lactate conversion inside the tumor. Once again, no significant differences between these rates for the two pancreatic tumor models,could be determined (Figure 4B).CONCLUSIONS
DMI’s potential for revealing pancreatic cancer in animal models by following 2H6,6’-glucose injections, was here demonstrated. Perfusion into the pancreatic tumors was successfully mapped, andmetabolic transformation into water and lactateas resulting from Krebs and glycolytic pathways followed.Acknowledgements
Support from the Israel Science Foundation, the Kimmel and the Clore Institutes for Magnetic Resonance (Weizmann Institute), the Thompson Family Foundation and the Israel Cancer Research Foundation, are acknowledged.References
(1) M. Lu, X. H. Zhu, Y. Zhang, G. Mateescu, W. Chen, Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37, 3518-3530 (2017).
(2) H. M. De Feyter, K. L. Behar, Z. A. Corbin, R. K. Fulbright, P. B. Brown, S. McIntyre, T. W. Nixon, D. L. Rothman, R. A. de Graaf, Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Science Advances 4, eaat7314 (2018).
(3) F. Kreis, A. J. Wright, F. Hesse, M. Fala, D.-e. Hu, K. M. Brindle, Measuring Tumor Glycolytic Flux in Vivo by Using Fast Deuterium MRI. Radiology 294, 289-296 (2019).
(4) M. J. Riis-Vestergaard, C. Laustsen, C. Ø. Mariager, R. F. Schulte, S. B. Pedersen, B. Richelsen, Glucose metabolism in brown adipose tissue determined by deuterium metabolic imaging in rats. International Journal of Obesity 44, 1417-1427 (2020).
(5) R. A. de Graaf, A. D. Hendriks, D. W. J. Klomp, C. Kumaragamage, D. Welting, C. S. Arteaga de Castro, P. B. Brown, S. McIntyre, T. W. Nixon, J. J. Prompers, H. M. De Feyter, On the magnetic field dependence of deuterium metabolic imaging. NMR in Biomedicine 33, e4235 (2020).
(6) C. von Morze, J. A. Engelbach, T. Blazey, J. D. Quirk, G. D. Reed, J. E. Ippolito, J. R. Garbow, Comparison of hyperpolarized 13C and non-hyperpolarized deuterium MRI approaches for imaging cerebral glucose metabolism at 4.7T. Magnetic Resonance in Medicine 2020;00:1-10.
(7) R. P. Martinho, Q. Bao, S. Markovic, D. Preise, K. Sasson, L. Agemy, A. Scherz, L. Frydman, Indentification of variable stages in murine pancreatic tumors by a multiparametric approach employing hyperpolarized 13C MRSI, 1H diffusivity and 1H T1 MRI. NMR in Biomedicine, e4446 (2020).
Figures