0921
Iron oxide-based Enzyme Mimic Nanocomposite for Dual-Modality Imaging Guided Chem-phototherapy and Anti-tumor Immunity Against Breast Cancer1Department of Biomedical Engineering, Guangzhou Medical University, Guangzhou, China, 2School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou, China, 3Siemens Healthineers, Shanghai, China, 4Philips Healthcare, Guangzhou, China
Synopsis
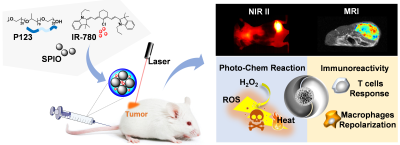
Due to the high incidence and mortality, breast cancer has become the major cause of cancer death among female. This study reports a kind of functional nanocomposite, which was designed to comprises Pluronic P123 to self-assembly superparamagnetic iron oxide nanocrystals (SPIONs) and IR-780 dyes into one system, exhibiting NIR-II and MR dual-modal imaging guided chem-phototherapy and anti-tumor immunity against triple-negative breast cancer.
Abstract
PURPOSE:This nanocomposite we manufactured could diagnose the tumor and confirm the time window of treatment via dual-modal imaging, and effectively suppress tumor growth along with metastasis thought chem-phototherapy and anti-tumor immunity.
METHODS:
Preparation and characterization of SPIO based nanocomposite (SPIO@NC)
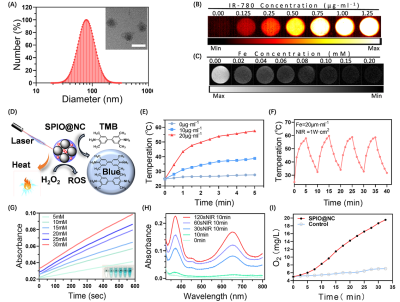
Firstly, SPIONs were synthesized through a high-temperature solvent thermal decomposition as reported previously [1, 2]. Secondly, SPIO based nanocomposite (SPIO@NC) was obtained though ultrasonic self-assembly towards Pluronic P123, SPIONs and IR-780 dyes into one system. The site and morphology of SPIO@NC was characterized by DLS and TEM. Furthermore, to evaluate stability of SPIO@NC, the diameter and the zeta potential were measured by several time points (1d, 6d, 11d, 16d, 21d, 30d). Besides, NIR imaging property of SPIO@NC sample was tested by second near-infrared imager; T2 relaxation time of SPIO@NC was measured to detect MRI properties of SPIO@NC using 1.5 T MRI system (sequence parameters: TR=10000ms, and TE=7.73ms). To evaluate the photothermal effect, SPIO@NC samples of different iron concentrations were exposed continuous laser for 5 minutes, respectively. Similarly, a swinging experiment of repeated heat absorption and heat dissipation were applied to verify the stable of photothermal therapy. Moreover, enzyme-like activity of SPIO@NC was detected by the ultraviolet spectrums of the reaction product (·OH) and the generation of oxygen (O2).
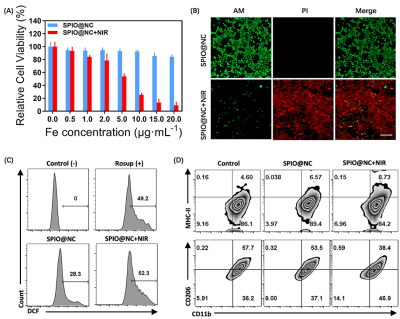
Biocompatibility, PTT, PDT and macrophage polarization in vitro
The photothermal effect of SPIO@NC at cellular level was proceeded by CCK-8 assay and propidium iodide (PI) and calcien-AM staining. To appraise the PDT effect, ROS level towards 4T1 cells with different treatments was measured using Flow cytometry analyses. To investigate the ability of SPIO@NC to repolarize M2 macrophages to M1 phenotype, the RAW264.7 cells were incubated with interleukin-4 (IL-4) for 24h to polarize macrophages toward M2 state, and cells were incubated with SPIO@NC or PBS for another 24h. After received with or without 2min 808nm laser irradiation and continued another 24h incubation, the population of M1 (MHC-II+CD11b+) and M2 (CD206+CD11b+) in RAW264.7 cells were estimated by flow cytometry.
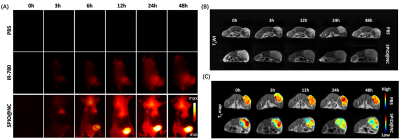
MRI-NIRF dual-modality imaging in vivo
Breast cancer model was established via Balb/c mouse (female, 6 weeks old) injected 4T1 cells. When the tumor volume reached to 1000 mm3 approximately, the tumor-bearing mice were divided into three groups (PBS, free IR-780 and SPIO@NC, n=3/group) in random to be taken NIRF II imaging. MR imaging in vivo of two groups mice (PBS group and SPIO@NC group, n=3/group) was performed using a clinical 3.0T MR system (GE, America). The T2-weighted and T2 MAP images of tumors were obtained before injection and post injection (0h, 3h, 12h, 24h, 48h). The T2-weighted images operation parameters were as follow: TR/TE: 3180/85ms; T2MAP images operation parameters: TR: 2800ms, TE: 10.1ms, 20.2ms, 30.4ms, 40.5ms, 50.6ms, 60.7ms, 70.8ms, 81.0ms.
Chem-phototherapy and Anti-tumor Immunity synergistic therapy in vivo
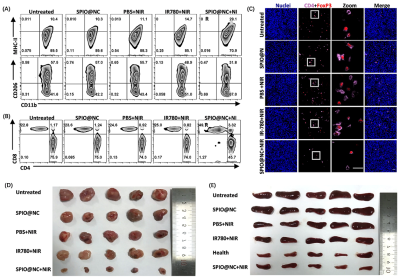
Firstly, the mice were classified as six groups randomly (n=5) as the tumors grew to approximately 100 mm3: ①health (no tumor), ②untreated, ③SPIO@NC, ④PBS+808nm NIR, ⑤IR-780+808nm NIR, ⑥SPIO@NC+808nm NIR. At 24h post injection with PBS, IR-780 and SPIO@NC, mice of group ④⑤⑥ were irradiated with 808 nm laser (1.0 W·cm-2, 5 min). The single-cell suspensions of tumors were gathered at 7th days and were stained with FITC anti-CD45, PC5.5 anti-CD11b, PE anti-MHC-II and PE/Cy 7 anti-CD206 antibodies for macrophage phenotype statistics, along with stained with FITC anti-CD45, PC5.5 anti-CD3, APC anti-CD4 and PE anti-CD8 and analyzed by flow cytometry. In addition, Treg cells (CD4+FOxP3+) in tumors were detected by immunofluorescence. After above treatments, the tumors and spleens were collected at 14th days. All statistical analyses of the measured data were performed using the software GraphPad Prism 8.0 (*p < 0.05, **p < 0.01).
RESULTS:
SPIO@NC system showed about 82±6.11 nm diameter size and the TEM images further confirmed the core-shell spherical shape (Fig. 1A), which showed great potential for tumor accumulation through the EPR effect. Fig. 1B&C showed remarkable NIR II and MRI imaging potential of SPIO@NC in equate solution. Excellent photothermal effect, nano-enzyme like catalysis and generation of O2 of SPIO@NC based NIR II irradiation were demonstrated in Figs. 1E-I. Furthermore, evidence for photothermic effect in 4T1 cells was in Figs. 2A-B. The capacity of generation of ROS and reprogram M2 macrophage to M1 macrophage were identified from Figs. 2C-D. Moreover, Fig. 3 illustrated outstand imaging performance of NIR II imaging and MRI of SPIO@NC. Fig. 4A-C exhibited the immunotherapeutic effect of SPIO@NC based phototherapy, upregulating the antitumor immune cells (M1 macrophage and CD8+ T cells) along with downregulating the protumor immune cells (M2 macrophage and Treg cells). Finally, compared with the control groups, tumors and spleens in SPIO@NC+NIR group shrank significantly (Fig. 4D&E).
CONCLUSION:
We developed a multifunctional SPIO@NC nanocomposite, which could diagnose the tumor and confirm the time window of treatment via NIRI/MRI dual-modal imaging, and effectively suppress tumor growth thought chem-phototherapy and anti-tumor immunity. This iron oxide-based nanocomposite provided a promising strategy for cancer theranostic applications.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC 8174100199,81971607), the Key Areas Research and Development Program of Guangdong (2019B020235001), the Science and Technology Program of Guangzhou (201804010101), Science and Technology Project of Yantian District in Shenzhen City, Guangdong Province, China (20190106), Sun Yat-sen University Young Teacher Training Project (20lgpy07).References
[1]. Sun, S., et al., Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. Journal of the American Chemical Society, 2004. 126(1): p. 273-279.
[2]. Gao, L., et al., Efficacy of MRI visible iron oxide nanoparticles in delivering minicircle DNA into liver via intrabiliary infusion. Biomaterials, 2013. 34(14): p. 3688-3696.
Figures