0919
Hyaluronan depletion improved intratumor pO2 and sensitized tumor to radiation therapy in pancreatic cancer model mouse.1Radiation Biology Branch, National Cancer Institute, Bethesda, MD, United States, 2Laboratory of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Saitama, Japan
Synopsis
PEGylated human hyaluronidase (PEGPH20) has been developed to enzymatically deplete tumor hyaluronan. The purpose of this study is to investigate physiologic and metabolic changes in pancreatic adenocarcinoma xenograft after PEGPH20 treatment by using multi-modal imaging and further determine the utility of PEGPH20 as radiosensitizer. PEGPH20 significantly increased intratumor pO2 and blood volume and decreased glycolytic flux assessed by EPRI, USPIO-MRI, and hyperpolarized 13C-MRI, respectively. PEGPH20 also enhanced treatment effect of radiotherapy in vivo. The results validated the utility of the imaging methods to non-invasively monitor changes in the tumor microenvironment and predicted the radiosensitizing effect upon hyaluronan depletion.
Purpose
In pancreatic ductal adenocarcinoma which is characterized by an intense desmoplastic feature, the extracellular matrix (ECM) can significantly influence the tumor microenvironment (TME) of cancer. Hyaluronan (HA), a major component of ECM, is associated with elevated tumor pressure (tP), vascular collapse, and poor perfusion in the TME conferring hypoxia (1,2). HA expression is also correlated with poor prognosis in the patients with PDACs (3). PEGylated human hyaluronidase (PEGPH20) has been developed to enzymatically deplete tumor HA and re-expand the tumor vasculature resulting in increased delivery of therapeutic molecules into the tumor (4,5). Although the phase 3 HALO 301 study, which compared nab-paclitaxel/gemcitabine with/without PEGPH20 in previously untreated patients with stage IV pancreatic cancer (6), failed to show the clinical benefit of PEGPH20, the impact of improved oxygenation by PEGPH20 on radiotherapy is still additional interest because the oxygen is known as a radio-sensitizer (7). The aim of this study was to investigate the changes in physiologic and metabolic profiles of the tumor in response to treatment with PEGPH20 using multi-modal imaging techniques. We also investigated the capability of PEGPH20 to enhance treatment effect of radiotherapy.Methods
Animal Study: BxPC3 (human pancreatic adenocarcinoma) cell line transduced with hyaluronan synthase 3 (HAS3) and PEGPH20 were given from Halozyme, Inc. Athymic nude mice were inoculated with 2 x 106 BxPC3-HAS3 tumor cells adjacent to the right tibial periosteum. For imaging experiments, approximately 600 mm3 tumor bearing mice were injected i.v. with 1 mg/kg of PEGPH20 on day 0 and day 3. Tumor bearing mice in the control group were injected with same amount of API buffer. All imaging experiments were performed at the timing of pre-treatment (before the first injection on day 0) and post-treatment (3 h after the 2nd PEGPH20 injection on day 3). For the experiment for radiotherapy, approximately 600 mm3 tumor was irradiated at 2.5Gy, q3d. PEGPH20 was administrated 3 hours prior to radiotherapy.EPRI: Parallel coil resonators tuned to 300 MHz were used. OX063 (1.125 mmol/kg) was injected i.v. to a mouse. FID signals were collected following the radiofrequency excitation pulses (60 ns) with a nested looping of the x, y, and z gradients, and each time point in the FID underwent phase modulation, enabling 3D spatial encoding. The repetition time was 8.0 µs. The number of averages was 4,000. After EPRI measurement, corresponding anatomic T2-weighted MR images were collected with a 1 T scanner (Bruker).
Blood volume imaging: MRI scanning was conducted on a 3 T scanner controlled with ParaVision 6.0 (Bruker). For blood volume (BV) calculation, spoiled gradient echo sequence images were collected before and 5 minutes after injection of ultra-small superparamagnetic iron oxide (USPIO) contrast (1.2 μL/g of body weight). The imaging parameters included the following: TE = 3.5 ms; and TR = 200 ms; FOV = 28 x 28 mm; matrix = 192 x 192.
Hyperpolarized 13C MRI: [1-13C] pyruvic acid (30 μL) containing 15 mmol/L Ox063 and 2.5 mmol/L of the gadolinium, ProHance (BraccoDiagnostics) was polarized in Hypersense DNP polarizer (Oxford Instruments). Hyperpolarized solution was rapidly injected i.v. to a mouse. MRI scanning was performed on a 3 T scanner (MR SOLUTIONS) using a 17 mm home-built 13C solenoid coil placed inside of a saddle coil tuned to 1H frequency. 13C spectra was acquired every 1 s for 240 s from the whole leg with a tumor. TR, spectral width, flip angle, and number of average were 1000 ms, 3300 Hz, 10°, and 1, respectively.
Results
EPRI showed significantly increased pO2 in PEGPH20 treated group (p = 0.0008, Fig. 1). Intratumor blood volume significantly increased in PEGPH20 treated group (p = 0.0045, Fig. 2). Hyperpolarized 13C-MRI with [1-13C] pyruvate showed significantly decreased lactate–to-pyruvate ratio in PEGPH20 treated tumor (p = 0.0046, Fig. 3). Either PEGPH20 monotherapy or radiotherapy delayed tumor growth compared to control buffer treated group. Combination of radiotherapy and PEGPH20 synergistically delayed tumor progression and prolonged the survival (Fig. 4).Conclusion
This study examined the effect of PEGPH20 on tumor microenvironment in xenograft model by using non-invasive multimodal imaging techniques. The results showed that depleting stromal HA by PEGPH20 improved blood perfusion, leading to improved oxygenation and the decreased pyruvate to lactate flux in the tumor. PEGPH20 potentially enhanced treatment effect of radiotherapy. These data support the effect of PEGPH20 on tumor microenvironment and propose an adjuvant use of PEGPH20 to enhance radiotherapy.Acknowledgements
No acknowledgement found.References
(1) Singha NC, Nekoroski T, Zhao C, et al. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol Cancer Ther 2015;14(2):523-32.
(2) Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62(1):112-20.
(3) Cheng XB, Sato N, Kohi S, et al. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PloS one. 2013;8(11):e80765.
(4) Thompson CB, Shepard HM, O'Connor PM, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther 2010;9(11):3052-64.
(5) Li X, Shepard HM, Cowell JA, et al. Parallel Accumulation of Tumor Hyaluronan, Collagen, and Other Drivers of Tumor Progression. Clin Cancer Res. 2018;24(19):4798-4807.
(6) Doherty GJ, Tempero M, Corrie PG. HALO-109-301: A Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future oncology (London, England). 2018;14(1):13-22.
(7) Axelson H, Fredlund E, Ovenberger M, et al. Hypoxia-induced dedifferentiation of tumor cells--a mechanism behind heterogeneity and aggressiveness of solid tumors. Seminars in cell & developmental biology. 2005;16(4-5):554-63.
Figures
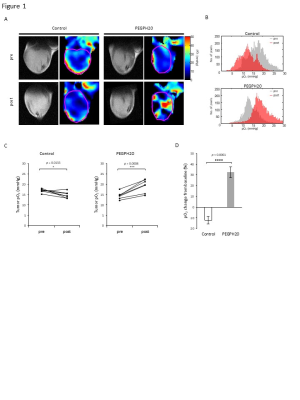
Fig. 1 EPR imaging showed increased pO2 after PEGPH20 treatment.
EPR imaging was performed in BxPC3-HAS3 tumor bearing mice treated with control buffer or PEGPH20 before and after treatment. A, Representative oxygen map obtained by EPR imaging and T2-weighted anatomical image. B, Histograms of pO2 distribution within the tumor of each group. C, The mean pO2 of each tumor shown by treatment group. D, pO2 change (%) from the baseline (pre-treatment) of each group.
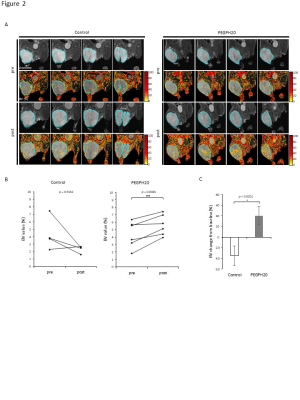
Fig. 2 UPIO MRI showed increased blood volume after PEGPH20 treatment.
MRI using USPIO was performed in BxPC3-HAS3 tumor bearing mice treated with control buffer or PEGPH20 before and after treatment. A, Representative imaging of USPIO intensity overlaid on T2-weighted anatomical image. Center three slices of each tumor are shown. B, Blood volume (%) of region of interest in each tumor (blue line in Fig.3A) are shown by treatment group. C, Blood volume change (%) from the baseline (pre-treatment) of each group.
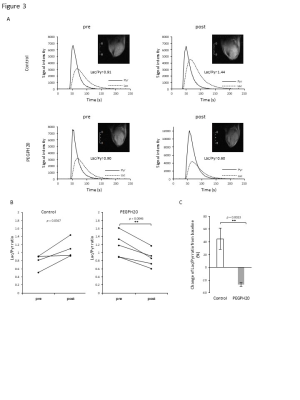
Fig. 3 HP [1-13C] Pyruvate MRI showed decreased Lac/Pyr ratio after PEGPH20 treatment.
HP [1-13C] Pyruvate MRI was performed in BxPC3-HAS3 tumor bearing mice treated with control buffer or PEGPH20 before and after treatment. A, Representative kinetics of [1-13C] pyruvate and [1-13C] lactate and its anatomical 1H image. B, Lac/Pyr ratio of each tumor are shown by treatment group. Individual values are shown. C, Lac/Pyr ratio change from the baseline (pre-treatment) of each group.
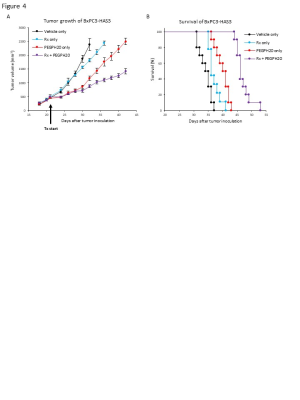
Fig. 4 Tumor growth and survival of radiotherapy + PEGPH20 treatment.
BxPC3-HAS3 inoculated mice were treated with either control buffer, PEGPH20 monotherapy, mono-radiotherapy, or radiotherapy + PEGPH20. A, Growth kinetics of each group. Data are shown as mean SE at each time point. B, Kaplan Meier survival curve of each group. Survival refers to the time before reaching the maximally allowed tumor volume of 2,500 mm3.