0906
Zero Echo Time Imaging Using Low Resolution k-Space Interleaves
Hanna Frantz1, Thomas Huefken1, Patrick Metze1, Kilian Stumpf1, Tobias Speidel1, and Volker Rasche1
1Department of Internal Medicine II, Ulm University Medical Center, Ulm, Germany
1Department of Internal Medicine II, Ulm University Medical Center, Ulm, Germany
Synopsis
Zero echo time (ZTE) imaging is an established approach for three-dimensional imaging of tissues and materials with ultrashort T2* relaxation times. An intrinsic disadvantage of this approach are missing data points within the center region of k-space due to hardware limitations. These missing points are often retrospectively interpolated or subsequently acquired using single-point imaging approaches or additional ZTE acquisitions with decreased resolutions. This abstract presents an approach that relies on the interleaved combination of ZTE read-outs with different resolutions combined with a Compressed Sensing reconstruction, to acquire more information around the k-space center, for high-quality ZTE without prolonged acquisition times.
Introduction
Magnetic resonance imaging (MRI) of tissues or samples with ultrashort T2* relaxation times such as e.g. lung1 or bone2 requires imaging approaches with short echo times (TE). Zero echo time (ZTE) enables data acquisition with almost negligible TE by application of the excitation pulse during the presence of a static read-out gradient. Since data cannot be acquired during playing out the RF pulse, missing data points in the center of k-space are one of the major limitations of ZTE, especially on clinical scanners. Several approaches have been introduced to acquire or estimate missing data points3, including algebraic reconstruction of oversampled data, or additional acquisitions based on low-resolution ZTE or Cartesian single-point imaging sequences. While algebraic reconstructions are limited to dead time durations of a few Nyquist dwells4, additional acquisitions lead to prolonged scan durations and therefore to an increased patient discomfort. We have introduced a concept for interfacing an independent receive unit to a clinical scanner, thus minimizing front-end switching times down to some µs5. However, the rather long excitation pulses on clinical systems still limit the application of ZTE even with T/R switch time in the µs range. The proposed method exploits the concept of the ZTE sequence. But instead of performing additional acquisitions, every n-th gradient is played out with decreased amplitude (lower resolution), to ensure coverage of the k-space center. A final acquisition without gradients measures the DC component (k0) in the steady-state. Non-linear reconstruction techniques are used to compensate aliasing artefacts.Methods
All images were acquired with a 3 T whole-body clinical imaging system (Ingenia 3.0T CX, Philips Healthcare, Best, The Netherlands) with an independent spectrometer (MRI Console Cameleon, RS2D, Mundolsheim, France), allowing for faster T/R switching, as well as for higher readout bandwidths. A custom-built hexagonal shaped single-loop coil with a side length of 40 mm was used for signal reception. The proposed sequence is based on a kooshball trajectory6 and FID sampling but modified such that each n-th read-out gradient is executed with ¼ of its initial amplitude. The lower resolution k-space data is used to fill the sphere around the k-space center, consisting of 2 missing k-space points in read-out direction for the given setup, which would remain unsampled in the case of a standard ZTE acquisition. Since no additional read-outs are added, the low-resolution gradients introduce minor undersampling to the outer regions of k-space. The occurring artefacts can easily be compensated by a Compressed Sensing (CS) reconstruction7. For image reconstruction, an in-house built reconstruction framework, implemented in MATLAB (The MathWorks, Natick, Massachusetts, USA) was used. The k-space density functions were calculated using a Voronoi tessellation8. The suggested approach was evaluated on a rubber phantom (60 x 53 mm) immersed in saline solution and initially in-vivo, by measuring the wrists of three healthy volunteers after obtaining written informed consent. All relevant scan parameters are listed in table 1.Results
Figure 1 shows images of the rubber phantom, acquired with a gradient strength of 15 mT/m, (A, B) and 24 mT/m (C, D), with (A, C) and without (B, C) lower resolution gradients. Figure 2 shows coronal (A,B) and sagittal (C,D) slices of the phantom, acquired with a gradient strength of 24 mT/m, with lower resolution gradients replacing every 25-th gradient. Images A and C were reconstructed using gridding and a direct FFT, whereas images B and D were reconstructed using a Compressed Sensing reconstruction combined with TV sparsity transform. The bright area above the phantom is caused by the coil itself. Figure 3 shows the in vivo images of a wrist, acquired using a standard ZTE trajectory without dead time correction for a gradient amplitude of 15 mT/m (A) and 24 mT/m (C) and with the modified approach including the low-strength gradients for a gradient amplitude of 15 mT/m (B) and 24 mT/m (D). Figure 4 shows coronal (A,B) and transversal (C,D) slices of a wrist, acquired with a gradient strength of 24 mT/m, with lower resolution gradients replacing every 25-th gradient. Images A and C were reconstructed using gridding and a direct FFT, whereas images B and D were reconstructed using a Compressed Sensing reconstruction combined with TV sparsity transform. The bright area above the wrist is caused by the coil itself. Adverse effects of more missing points around the k-space center can clearly be appreciated in case of the 24 mT/m gradient amplitude.Discussion and Conclusion
Replacing ZTE read-outs with lower resolution gradients yields useful additional information within the center region of k-space without any increase in total scan duration. The resulting undersampling of the outer region of k-space does not introduce adverse aliasing artefacts, but rather artefacts which can easily be compensated by a CS reconstruction. The introduction of the lower resolution read-outs becomes increasingly important with increasing gradient strength, since more k-space center points remain unsampled. The presented approach yields qualitative ZTE images within the same acquisition duration as a standard ZTE acquisition since no additional acquisitions are required. The concept of lower resolution gradient can furthermore be used to additionally acquire k0 at every m-th interleave to derive a gating signal for e.g. lung imaging, which is a dedicated application for ZTE imaging.Acknowledgements
The authors thank the Ulm University Center for Translational Imaging MoMAN for its support. This work was supported by DFG RA1660/9-1. Technical support from Philips Healthcare is gratefully acknowledged.References
- Wild JM, Marshall H, Bock M, et al. MRI of the lung (1/3): methods. Insights Imaging. 2012;3(4):345-53.
- Wehrli FW. Magnetic resonance of calcified tissues. J Magn Reson. 2013;229:35-48.
- Froidevaux R, Weiger M, Brunner DO, et al. Filling the dead-time gap in zero echt time MRI: Principles compared. Magn Reson Med. 2018;79(4):2036-2045.
- Weiger M, Brunner DO, Tabbert M, et al. Exploring the bandwidth limits of ZTE imaging: Spatial response, out-of-band signals, and noise propagation. Magn Reson Med. 2015;74(5):1236-47.
- Eder M, Horneff A, Paul J, et al. A Signal Acquisition Setup for Ultrashort Echo Time Imaging Operating in Parallel on Unmodified Clinical MRI Scanners Achieving an Acquisition Delay of µs. IEEE Trans Med Imaging. 2020;39(1):218-225.
- Wong ST, Roos MS. A strategy for sampling on a sphere applied to 3D selective RF pule design. Magn Reson Med. 1994;32(6):776-84.
- Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-95.
- Rasche V, Proksa R, Sinkus R, et al. Resampling of data between arbitrary grids using convolution interpolation. IEEE Trans Med Imaging. 1999;18(5):385-92
Figures
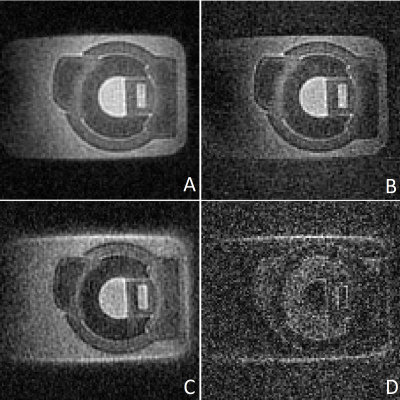
Figure 1: Coronal slice of a dedicated rubber phantom acquired at 15
mT/m gradient strength (A, B) and 24 mT/m (C, D). Images A and C were
acquired with the modified approach including the low-strength gradients.
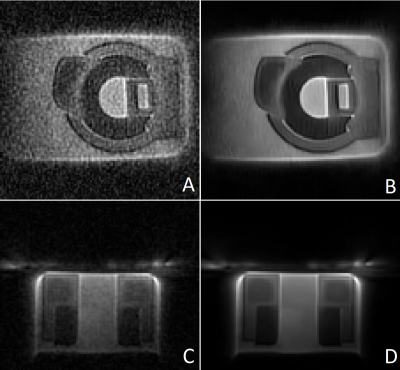
Figure 2: Coronal (A, B) and sagittal (C, D) slices of a rubber phantom acquired with a modified ZTE sequence at 24 mT/m
including low-strength gradients. Images B and D were reconstructed using a
Compressed Sensing reconstruction. The bright area above the phantom in the
sagittal views is caused by the coil itself.
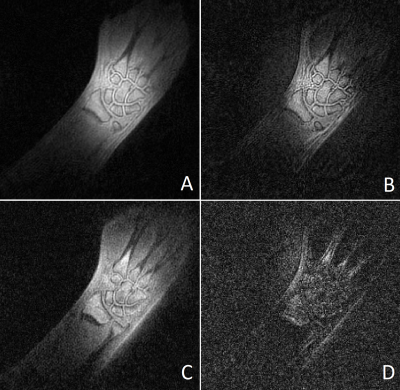
Figure 3: Coronal slice of a wrist
acquired at 15 mT/m gradient strength (A, B) and 24 mT/m (C, D). Images A
and C were acquired with the modified approach including the low-strength
gradients.
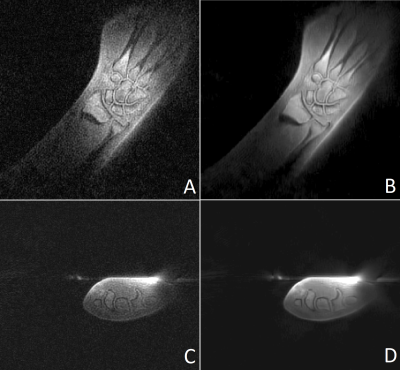
Figure 4: Coronal (A, B) and transversal (C, D) slices of a wrist acquired with a modified ZTE sequence at 24
mT/m including low-strength gradients. Images B and D were reconstructed using
a Compressed Sensing reconstruction. The bright area above the phantom in the transversal views is caused by the coil itself.
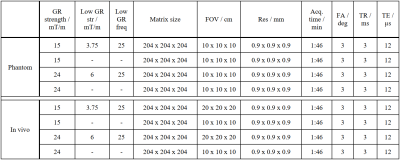
Table 1: Scan parameters including gradient strength, low resolution gradient strength, low resolution gradient frequency, matrix size, field of view (FOV), resolution, acquisition duration, flip angle (FA), repetition time (TR) and echo time (TE).