0871
Clinical application of ASL-based non-invasive perfusion territory mapping and time-resolved angiography in cerebrovascular diseases1School of Medicine, Department of Neuroradiology, Technical University of Munich (TUM), Munich, Germany, 2Philips Healthcare, Horgen, Switzerland, 3Philips Healthcare, Hamburg, Germany, 4Philips Healthcare, Best, Netherlands, 5Philips Japan, Tokyo, Japan, 6University Hospital Schleswig-Holstein Campus Kiel, Kiel, Germany, 7Department of Radiology, University Ulm Medical Center, Ulm, Germany
Synopsis
Imaging of blood supply patterns is of high clinical interest to improve diagnostics in cerebrovascular diseases (CVD). However, the only clinically available imaging method is invasive digital subtraction angiography (DSA). We present data from 17 participants, including 7 CVD patients, with non-invasive MRI-based perfusion territory mapping by super-selective arterial spin labeling (ss-ASL) and time-resolved angiography by 4D-sPack with automated planning. Our results successfully confirmed automated planning even in CVD patients. Collateral pathways were non-invasively depicted by ss-ASL and 4D-sPack. Moreover, congruent results with DSA, contrast-enhanced MR-angiography and perfusion MRI demonstrated great potential for future routine application of ss-ASL and 4D-sPack.
Purpose
Cerebrovascular diseases (CVD) are a main cause of death in developed countries.1 CVDs often relate to an increased stroke risk, but collateral blood supply is known as an important protective vascular pathway, which can prevent strokes.2 Detection of vascular territories and critical collateralization status could thus play an important role to improve diagnostics in CVDs and support delicate treatment decisions.1,3 However, collateral blood flow is highly individual4,5 and not commonly clinically evaluated. The only available method is invasive catheter angiography by digital subtraction angiography (DSA), which requires hospitalization, has complication risks, takes up to an hour, and applies high radiation dose (approximately 11 mSv per labeled vessel).6To enable widespread imaging of blood supply patterns, two non-invasive MR-based alternatives using arterial spin labeling (ASL) have been proposed. First, super-selective ASL (ss-ASL) enables perfusion territory mapping by individually labeling brain feeding arteries.7 Second, 4D ss-ASL combined with contrast-enhanced timing-robust angiography-keyhole and view-sharing (4D-sPack) enables selective time-resolved angiography.8 While previous ss-ASL applications were very promising,9-12 manual planning of the labeling spot is cumbersome, especially in altered vascular architectures, and limits widespread application. To improve clinical usability, an automated planning tool for ss-ASL has been proposed,13 but was neither applied in CVDs, nor combined with 4D-sPack so far.
The aim of our study was therefore to evaluate the clinical applicability of ss-ASL and 4D-sPack in two steps. First, we probed the automated planning in healthy controls. Second, we applied it in CVD patients in comparison with DSA, contrast-enhanced MR angiography, dynamic susceptibility contrast (DSC) and pseudo-continuous ASL (pCASL).
Methods
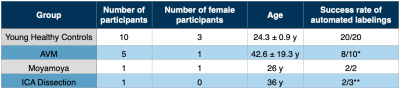
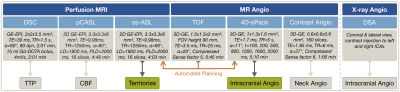
In this ongoing clinical study, 17 participants (10 young healthy controls and 7 CVD patients, including 5 arteriovenous malformation (AVM), 1 moyamoya and 1 internal carotid artery (ICA) dissection, see Tab.1) underwent MRI on a 3T Philips Ingenia Elition or Achieva (Philips Healthcare, Best, The Netherlands) on software release R5.6 with a custom patch. Measurements and derived parameters are summarized in Figure 1. Maps of cerebral blood flow (CBF) were derived from pCASL with 2000 ms post-label-delay.13 Labeling positions of ss-ASL and 4D-sPack at both ICAs and a vertebral artery (VA) were set by a fully automated tool integrated at the scanner console, based on time-of-flight (TOF) neck-angiography.14 Post-processing was performed with SPM1215 and custom-built Matlab (Mathworks, Natick, USA) programs. In selected patients, intracranial DSA, contrast-enhanced MR neck-angiography and DSC-based time-to-peak (TTP) were obtained.Results
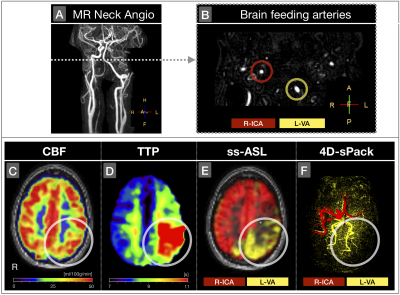
The automated planning tool successfully identified labeling positions at both ICAs in all healthy participants (Tab.1). In CVD patients, automated planning was successful in 12 of 15 vessels (ICAs and VA). Two failed attempts in the same patient were caused by head motion during ASL acquisition, while labeling positions seemed correctly identified. The third failed attempt was due an ICA dissection (Fig.2B).Despite of difficile cerebral blood supply in a patient with left-ICA dissection, occlusion of left first media segment and a hypoplastic right-VA, the CBF-map seemed almost normal (Fig.2C). Here, ss-ASL and 4D-sPack demonstrated how the two brain feeding right-ICA and left-VA mainly supply the brain, with takeover of left anterior and media supply by right-ICA (Fig.2E,F). Temporal perfusion characteristics by TTP showed severe delays in the left-VA territory (Fig.2D-F).
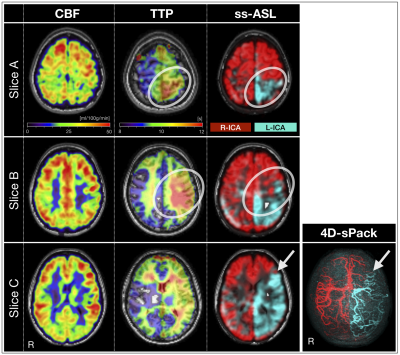
In a patient with moyamoya disease, perfusion mapping showed no very severe perfusion alternations (Fig.3). Labeling of both ICAs with ss-ASL and 4D-sPack depicted peripheral intracranial arterial branches and takeover by collateral vessels originating from frontal right-ICA branches. The anterior border-zone between left- vs. right-ICA territories from ss-ASL spatially coincided with the 4D-sPack angiogram. Furthermore, time-resolved 4D-sPack information depicted retrograde filling of the left media territory (Fig.4A) in agreement with delayed perfusion (Fig.4C). Comparisons of intracranial 4D-sPack angiograms by with DSA showed excellent spatial correspondence, capturing even distal small collateral vessels in the contralateral hemisphere (Fig.4B).
Discussion
As hypothesized, 4D-sPack and ss-ASL were successfully applied in CVD patients and took around 5 minutes per labeled vessel by each method. Non-invasive angiography by 4D-sPack depicted territorial blood supply and was confirmed by DSA, in agreement with a recent study.16 Even collateral supply of comparably small distal arterial branches was captured by 4D-sPack. Furthermore, temporal characteristics of interhemispheric supply patterns allowed to observe retrograde fillings, which is typical for collateral pathways in moyamoya disease.17 This agreed with elevated TTP, in line with the literature.2,17 At the same time, ss-ASL enabled visualization how CBF is widely spatially maintained in patients with severe blood supply disturbances.The automated labeling tool was successfully applied in CVD patients. In the few failed attempts, even manual labeling was unsuccessful. Associated motion artefacts could be reduced by carefully immobilizing the patient’s head. Besides application in CVDs,9,12,14 previous studies indicated great potential in stroke1,18,19 and brain tumor11 diagnostics as well as treatment planning1,20.
Conclusion
In conclusion, we demonstrated the high clinical potential of non-invasive perfusion territory mapping by ss-ASL and time-resolved angiography by 4D-sPack in CVD patients with whole brain coverage in clinically applicable scan time. The results agreed well with conventional invasive imaging methods. Moreover, 4D-sPack derived additional diagnostic value by temporal perfusion characteristics. In combination with automated labeling, both methods are highly promising and seem feasible as part of routine clinical MRI in primary diagnostic examination and follow-up scans.Acknowledgements
We acknowledge support by Friedrich-Ebert-Stiftung (grant to SK), Dr.-Ing. Leonhard-Lorenz-Stiftung (grant SK 971/19) and the German research Foundation (DFG, grant PR 1039/6-1).References
1. Donahue MJ, et al. Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. JCBFM 2018;38:1391-417.
2. Liebeskind DS. Collateral Circulation. Stroke 2003;34(9):2279-84.
3. Jung S, et al. Relevance of the cerebral collateral circulation in ischaemic stroke: time is brain, but collaterals set the pace. Swiss Med Wkly. 2017;147:w14538.
4. van der Zwan A, et al. A quantitative investigation of the variability of the major cerebral arterial territories. Stroke 1993;24(12);1951–9.
5. Zarrinkoob L, et al. Blood flow lateralization and collateral compensatory mechanisms in patients with carotid artery stenosis. Stroke 2019;50(5);1081–88.
6. Guberina N, et al. Dose comparison of classical 2-plane DSA and 3D rotational angiography for the assessment of intracranial aneurysms. Neuroradiology 2016;58(7):673-8.
7. Helle M, et al. Superselective pseudocontinuous arterial spin labeling. MRM 2010;64(3):777-86.
8. Obara M, et al. Improved selective visualization of internal and external carotid artery in 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK). MRM 2020;73:15-22.
9. Richter V, et al. MR Imaging of individual perfusion reorganization using superselective pseudocontinuous arterial spin-labeling in patients with complex extracranial steno-occlusive disease. AJNR 2017;38(4);703–11.
10. Helle M, et al. Superselective arterial spin labeling applied for flow territory mapping in various cerebrovascular diseases. JMRI 2013;38(2):496-503.
11. Jensen-Kondering U, el al. Non-invasive qualitative and semiquantitative presurgical investigation of the feeding vasculature to intracranial meningiomas using superselective arterial spin labeling. PLoS One 2019;14(4):e0215145.
12. Kaczmarz S, et al. Increased variability of watershed areas in patients with high-grade carotid stenosis. Neuroradiology 2018;60(3):311-23.
13. Alsop et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. MRM 2015;73(1):102–16.
14. Helle M, et al. Advanced automatic planning for super-selective arterial spin labeling flow territory mapping. Proc of ISMRM 2018, Paris, France, Abstract 302.
15. Statistical Parametric Mapping software (SPM12) Version 6225: www.fil.ion.ucl.ac.uk/spm. Wellcome Trust Centre, London, UK.
16. Togao O, et al. Vessel-selective 4D-MR angiography using super-selective pseudo-continuous arterial spin labeling may be a useful tool for assessing brain AVM hemodynamics. Eur Radiol 2020;30(12):6452-63.
17. Fan AP, et al. Long-Delay Arterial Spin Labeling Provides More Accurate Cerebral Blood Flow Measurements in Moyamoya Patients. Stroke 2017;48(9):2441-9.
18. van Laar PJ, et al. Brain perfusion territory imaging: methods and clinical applications of selective arterial spin-labeling MR imaging. Radiology 2008;246(2):354-64.
19. Hartkamp NS, et al. Arterial spin labeling magnetic resonance perfusion imaging in cerebral ischemia. Curr Op Neurol 2014;27(1):42-53.
20. Hartkamp NS, et al. Mapping of cerebral perfusion territories using territorial arterial spin labeling: techniques and clinical application. NMR in Biomed 2013;26(8):901-12.
Figures