0866
Beyond crossing fibers: investigating the prevalence of bottleneck configurations in the human brain white matter with diffusion tractography1Vanderbilt University Medical Center, Nashville, TN, United States, 2Universite de Sherbrooke, Sherbrooke, QC, Canada, 3CEA University of Bordeaux, Bordeaux, France, 4Cardiff University, Cardiff, United Kingdom, 5Vanderbilt University, Nashville, TN, United States
Synopsis
Bottleneck regions of the brain, or areas where multiple white matter pathways of the brain converge and subsequently diverge, present a challenge to anatomically accurate fiber tractography of the brain. In this work, we investigated the prevalence and locations of bottleneck regions. Our results indicate that most white matter contains multiple overlapping and crossing bundles. Moreover, individual orientations withina voxel are associated with multiple bundles, which represent bottlenecks. These findings have profound implications for tractography algorithms which aim to map unknown connections across the brain, and strengthen the awareness of limitations or challenges facing these image processing techniques.
Introduction
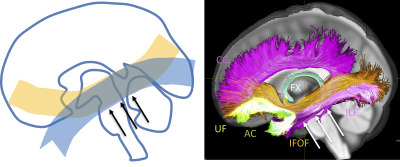
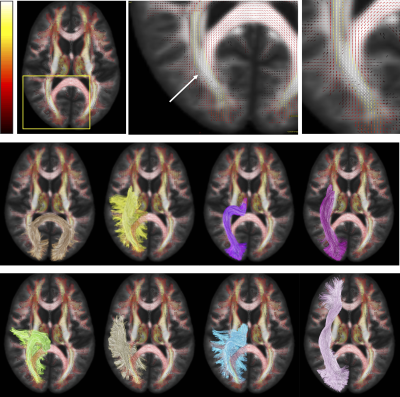
A major challenge to mapping anatomically accurate fiber bundles of the brain occurs when multiple white matter bundles converge towards a single region and share the same parallel orientation before diverging and continuing towards their final cortical or sub-cortical terminations (Figure 1) [1]. These so-called “bottleneck” regions contribute to the ill-posed nature of the tractography process, and lead to both false positive and false negative estimated connections. Yet, a thorough characterization of these regions has not been performed. The aim of this study is to quantify the prevalence and to describe the locations of bottleneck regions in the human brain.Methods
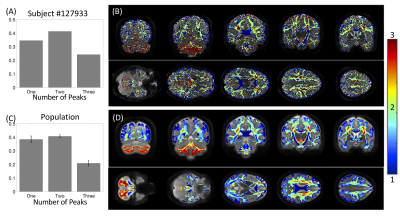
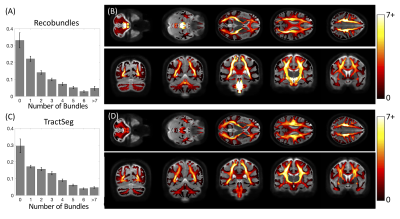
We utilized the data from 25 healthy subjects in the Human Connectome Project and reconstructed fiber orientation distributions using multi-shell multi-tissue constrained spherical deconvolution. We then ran two, common, automated pipelines for bundle extraction, Recobundles [2] and TractSeg [3], which generated 78 and 72 white matter bundles, respectively. These methods represent the most conservative approach to estimating bottlenecks by only including pathways anatomically-validated through dissection and segmented by neuroanatomists, thus representing a lower bound on bottleneck prevalence and magnitude.On the scale of voxels, we can describe the 3D volume of space as being a single-fiber voxel or crossing-fiber voxel based on the number of discrete peaks of the fiber orientation distribution (FOD), or number of fiber elements (fixels) in the FOD. We can also describe a voxel as being a single-bundle voxel or multi-bundle voxel based on the number of bundles traversing throughout the voxel. Finally, for each fixel, we can also classify as a single-bundle fixel (i.e., one bundle per direction) or multi-bundle fixel (i.e., multiple bundles in a single direction). These multi-bundle fixels represent bottleneck regions for tractography.
Results
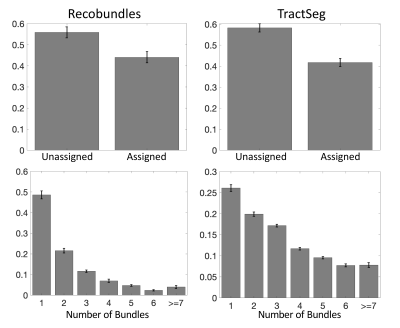
First, we confirm that a majority of voxels in the brain contain crossing fibers (Figure 2), in agreement with previous literature [4, 5]. Second, we find that a majority of voxels in the brain are sites for the passage of multiple bundles (Figure 3), with many regions indicating overlap of 7+ white matter bundles. Visually, we see a number of regions, particularly in small gyri or superficial U-fibers, which have not been assigned to bundles. Additionally, regions that contain only a single bundle are rare.Analyzing fixels (specific orientations within voxels), we find that most of the fixels of the brain are unassigned to a specific known bundle (Figure 4). Additionally, many fixels are associated with a number of bundles (some >7 bundles). Probing these bottleneck regions in detail, we identify regions of the brain that will present the most challenging problems for tractography algorithms, including the anterior-posterior oriented white matter of the occipital lobe (Figure 5), the superior-inferior oriented white matter of the brainstem, and the superior-inferior oriented white matter of the internal capsule.
Discussion
The convergence of bundles into a nearly parallel funnel, and subsequent divergence, may lead to a combinatorial number of possible pathways that tractography algorithms may choose to take, leading to the generation of possibly anatomically non-existent pathways. We find that voxels with multiple bundles are indeed highly prevalent throughout the brain. Taking this a step further, we find that a majority of individual FOD peaks within a voxel (i.e., fixels) are associated with multiple bundles. While this may not present a problem for bundle-specific tractography with the use of manually placed priors [6], bundle templates [7, 8], or machine learning [3, 9, 10], mapping the entirety of the human connectome ultimately strives to map allof the unique bundles of the brain. The identified bottleneck regions clearly present obstacles to this mapping when the true connections are not known a priori. From a whole-brain connectome, streamlines which pass through these fixels without explicit knowledge of their existence should be suspected to be false positive connections generated by the process. Above problems presented to connectomics studies, this also highlights the problems of proposing the existence of a new or unique pathway from diffusion MRI alone, even if the pathway is reproducible across scans and subjects.Interestingly, ~30% of the voxels, and ~50% of all fixels, of the white matter are not associated with a bundle, for both of the tractography segmentation methods. While many of these could be spurious peaks or isolated voxels in white matter, larger expanses of coherently oriented FODs could be regions which may represent underexplored white matter pathways which can eventually be added to our repertoire and collection of bundles.
In addition to tractography, the diffusion signal that results from a single fiber population (i.e., the fiber response function) has applications towards tissue microstructural modeling. Typically, this response function is derived from studying regions of low complexity, specifically, regions that are considered single fiber populations and contain only a single peak in the FOD [11-13]. Here, however, we have shown that even if a voxel or region contains a dominant orientation and high anisotropy, a majority of these regions are composed of multiple, distinct fiber pathways, that may have varying densities, sizes, and distributions of axons. Thus, these so-called single fiber regions are very often multi-bundle regions.
Acknowledgements
This work was supported by the National Institutes of Health under award numbers R01EB017230 (B.A.L), and T32EB001628 (K.G.S.), and in part by VICTR VR3029 and the National Center for Research Resources, Grant UL1 RR024975-01. CMWT was supported by a Sir Henry Wellcome Fellowship (215944/Z/19/Z) and a Veni grant (17331) from the Dutch Research Council (NWO).References
1. Maier-Hein, K.H., et al., The challenge of mapping the human connectome based on diffusion tractography.Nat Commun, 2017. 8(1): p. 1349.
2. Garyfallidis, E., et al., Recognition of white matter bundles using local and global streamline-based registration and clustering.Neuroimage, 2018. 170: p. 283-295.
3. Wasserthal, J., P. Neher, and K.H. Maier-Hein, TractSeg - Fast and accurate white matter tract segmentation.Neuroimage, 2018. 183: p. 239-253.
4. Schilling, K., et al., Can increased spatial resolution solve the crossing fiber problem for diffusion MRI?NMR Biomed, 2017. 30(12).
5. Jeurissen, B., et al., Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging.Hum Brain Mapp, 2013. 34(11): p. 2747-66.
6. Chamberland, M., et al., Active delineation of Meyer's loop using oriented priors through MAGNEtic tractography (MAGNET).Hum Brain Mapp, 2017. 38(1): p. 509-527.
7. Hansen, C.B., et al., Pandora: 4-D white matter bundle population-based atlases derived from diffusion MRI fiber tractography.bioRxiv, 2020: p. 2020.06.12.148999.
8. Rheault, F., et al., Bundle-specific tractography with incorporated anatomical and orientational priors.Neuroimage, 2019. 186: p. 382-398.
9. Poulin, P., et al., Tractography and machine learning: Current state and open challenges.Magn Reson Imaging, 2019.
10. Wasserthal, J., P.F. Neher, and K.H. Maier-Hein. Tract Orientation Mapping for Bundle-Specific Tractography. in Medical Image Computing and Computer Assisted Intervention – MICCAI 2018. 2018. Cham: Springer International Publishing.
11. Dhollander, T., D. Raffelt, and A. Connelly, Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. , in ISMRM Workshop on Breaking the Barriers of Diffusion MRI. 2016.
12. Tax, C.M., et al., Recursive calibration of the fiber response function for spherical deconvolution of diffusion MRI data.Neuroimage, 2014. 86: p. 67-80.
13. Tournier, J.D., F. Calamante, and A. Connelly, Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution.Neuroimage, 2007. 35(4): p. 1459-72.
Figures