0862
Ultra-quality 4D-MRI synthesis using deep learning-based deformable image registration1The Hong Kong Polytechnic University, Hong Kong, China, 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Radiation Oncology, Peking University Cancer Hospital & Institute, Beijing, China, 3The University of Hong Kong, Hong Kong, China
Synopsis
We have developed and validated an ultra-quality 4D-MRI synthesis technique using deep learning-based deformable image registrations. The displacement vector fields between breathing frames were obtained from low-quality 4D-MRI. They were then applied to high-quality stationary T1, T2, and diffusion weighted images to generate ultra-quality 4D-MRI. The synthetic 4D-MRIs were verified in terms of tumor motion accuracy and image quality. All the motion errors were in a sub-voxel level, and the image quality was significantly improved. This technique holds great potential in volumetric tumor tracking with high accuracy.
Introduction
The position of liver tumors is heavily affected by respiratory motions, which brings great difficulties on target delineation in radiation treatment (RT). Four-dimensional magnetic resonance imaging (4D-MRI) has shown great potential in image guidance in RT. However, there is always a trade-off between image quality and efficiency. Due to limited hardware and imaging time, 4D-MRI usually suffers from inconsistent and insufficient tumor contrast and severe motion artifacts.1 One solution to generate high-quality 4D images is to obtain organ motion patterns from low-quality 4D images and apply them to static high-quality 3D images using deformable image registration (DIR).2 However, the long computation time in conventional iterative DIR algorithms limits its application on tumor tracking in IGRT where short data processing time is required. As deep learning (DL) grows rapidly in recent years, some studies verified the feasibility of generating displacement vector fields (DVFs) with DL models in a second.3 In this study, a DL model was proposed for 4D-MRI DIR that extracts inter-frame motion patterns, which were then applied to high-quality 3D MRIs to synthesize ultra-quality 4D-MRI for MR-guided RT.Methods
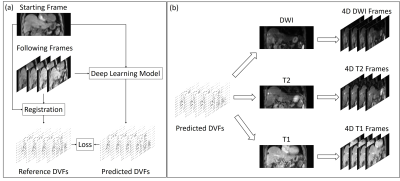
Twenty-seven patients were collected from Beijing Cancer Hospital with IRB approval. Seventeen patients were used for training and the remaining ten patients were used for validation. Each patient received a T1-weighted (T1w) 4D MRI scan using TWIST volumetric interpolated breath-hold examination (TWIST-VIBE) sequence. The scanning parameters of the TWIST-VIBE sequence are TR = 3.44 ms, TE = 1.23/2.46 ms, flip angle = 5o, bandwidth = 1420 Hz, matrix size = 160x128, and the temporal resolution was 0.69s per frames. The 4D-MRI scans were continuous and typically had 72 volumetric frames. In addition, each patient received multi-parametric 3D MR scans, including T1w, T2-weighted (T2w), and diffusion-weighted MR imaging (DWI) with b value of 50 and 800. The voxel size of the T1w 4D-MRI was 2.7x2.7x5.0 mm3 and 1.2x1.2x3.0, 1.5x1.5x5.0, and 1.5x1.5x6.0 mm3 for the T1w, T2w, and DWI images, respectively.An illustration of the training and application of the DL DIR model was shown in Figure 1. The DL model utilized in this study was a VoxelMorph-based model,3 which received concatenated moving and fixed image pairs and outputted predicted DVFs. Training samples were prepared before the DL model training. Among the 72 frames of a 4D-MRI scan, the first frame was paired with all following frames as the moving and fixed images, making a total number of image pairs of 1728. For each image pair, a reference DVF was calculated using the conventional iterative approach with isotropic total variation constrain under the recommended parameter settings and used as the prediction target.4 The model training was supervised by the difference between reference and predicted DVFs and image dissimilarity. In application, the 4D-MRI frames at the same respiratory phases with 3D MRI scans were selected as moving images and registered to the first 20 frames of 4D-MRI. The obtained DVFs were then applied to the 3D MRI scan to synthesize ultra-quality 4D-MRI at corresponding respiratory phases. For each testing patient, tumor trajectories were tracked both in the original 4D-MRI and four synthetic 4D-MRIs and then compared to verify the motion accuracy. Tumor-liver contrast-to-noise ratio (CNR) and full width half maximum (FWHM) of the image edge spread function were calculated on the original 4D-MRI, synthetic 4D-MRIs, and 3D MRIs to quantify the image quality improvements.
Results
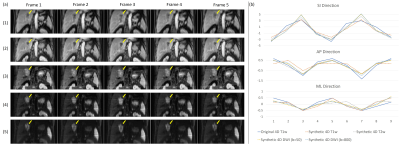
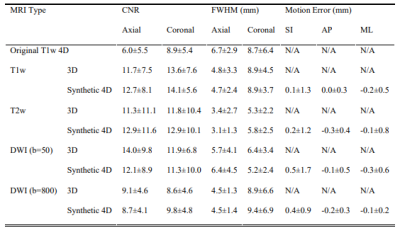
Visual comparison between the original T1w 4D-MRI and synthetic 4D-MRIs of one patient of the validation set was shown in Figure 2(a). The image quality of synthetic 4D-MRIs was largely improved, and the tumor showed better visibility in the synthetic 4D-MRIs. Figure 2(b) showed the well-agreed tumor motion trajectories between the original and synthetic 4D-MRIs. Detailed quantitative evaluation results of the validation set were listed in Table 1. The CNR and FWHM of the edge spread function in the synthetic 4D-MRIs were significantly higher than the original 4D-MRI while comparable with the 3D MRIs; the relative motion error of all synthetic 4D-MRIs was smaller than their voxel size. The temporal cost for the ultra-quality 4D-MRI synthesis was about 10ms/frame.Discussion
We developed a deep learning DIR-based ultra-quality 4D-MRI synthesis technique in this study. The image quality of the synthetic 4D-MRIs was significantly enhanced, and the tumor motions agreed well with the original ones. Compared to previous image enhancement methods, this technique enables multi-parametric 4D-MRI generation with much shorter processing time. Currently, 4D-MRI is rarely used in practice for its poor image quality. This technique enhanced its quality and makes it more practical in RT.Conclusion
A DIR-based ultra-quality 4D-MRI synthesis technique was developed using deep learning. The synthesized 4D-MRI showed accurate tumor motion trajectories with significantly improved image quality than the traditional 4D-MRI. This technique enables real-time accurate volumetric tracking of tumors and has great promise in IGRT.Acknowledgements
This research was partly supported by Hong Kong research grants(General Research Fund (GRF) from University Grants Committee: GRF 151021/18M and GRF 151022/19M; Health and Medical ResearchFund (HMRF) from Food and Health Bureau: HMRF 06173276).References
1. Liu Y, Yin F, Chen N, et al. Four dimensional magnetic resonance imaging with retrospective k‐space reordering: A feasibility study. Med Phys. 2015;42(2):534-41
2. Dang J, Luo O, Gu X, et al. Deformation vector fields (DVF)-driven image reconstruction for 4D-CBCT. J Xray Sci Technol. 2015;23(5):647
3. Balakrishnan G, Zhao A, Sabuncu M R, et al. VoxelMorph: a learning framework for deformable medical image registration. IEEE Trans Med Imaging. 2019;38(8):1788-1800
4. Vishnevskiy V, Gass T, Szekely G, Tanner C, et al. Isotropic total variation regularization of displacements in parametric image registration. IEEE Trans Med Imaging. 2017;36(2):385-395
Figures