0854
Real-Time Slice Steering for MR-Guided Interventions Using Endovascular Devices Equipped with Passive MRI Markers
Daniel Christopher Hoinkiss1, Han Nijsink2, Paul Borm3, Sabrina Haase1, Jan Strehlow1, Jurgen Futterer2, and Torben Pätz1
1Fraunhofer Institute for Digital Medicine MEVIS, Bremen, Germany, 2Radboud University Medical Centre (Radboudumc), Nijmegen, Netherlands, 3Nano4imaging GmbH, Aachen, Germany
1Fraunhofer Institute for Digital Medicine MEVIS, Bremen, Germany, 2Radboud University Medical Centre (Radboudumc), Nijmegen, Netherlands, 3Nano4imaging GmbH, Aachen, Germany
Synopsis
MR-guided endovascular interventions will enable widely distributed therapies to be performed without radiation. We present a workflow for interventional slice steering based on endovascular device tracking to increase the precision during MR-guided interventions. Merging real-time tracking information based on passive MRI markers with pre-calculated therapy planning allows the slice steering to automatically adjust itself to the predicted vessel pathways for smooth and accurate device monitoring.
Introduction
MR-guided interventions allow widely distributed therapies to be performed without radiation, one example being Percutaneous Transluminal Angioplasty (PTA) [1-2]. To support these procedures, we developed a platform including intervention planning and real-time intervention support. In previous work we presented a guidewire tracking algorithm that monitors the position of endovascular devices with high accuracy [3]. In this abstract we merge the tracking information with the intervention planning for real-time slice steering to follow the endovascular device during intervention.Methods
Three parallel MRI slices are used for three-dimensional wire tracking. A gradient echo sequence was designed for non-flow-dependent depiction of the guidewire which is equipped with passive MRI markers providing negative MRI artifacts (Nano4Imaging GmbH, Dusseldorf, Germany). Accelerated by common techniques like GRAPPA [4] and Partial Fourier [5], the sequence acquires the three slices in about 500ms with following sequence parameters: 300mm x 300mm FOV, 144 x 144 acquisition matrix, 5mm slice thickness, TE/TR = 1.7ms/3.4ms, 6° flip angle, 75% phase resolution, 6/8 partial Fourier and 3-fold integrated GRAPPA. The wire design, marker detection and wire reconstruction algorithms were presented in [3] and are summarized in Figure 1.The reconstructed guidewire is used to estimate position and orientation for real-time MRI slice steering. Since the reconstruction of the guidewire uses a second-degree polynomial, the fitting imaging plane can be calculated using simple plane equations with three sampling points (Fig. 2). A bilateral communication protocol is used such that the directions of the readout and phase-encoding axes are known prior to slice adjustment. The missing degree of freedom (in-plane orientation) is solved by automatically aligning the wire tip along the encoding direction that results in less rotation, which helps to reduce spin history artifacts as well as sudden direction changes. The plane center is then positioned at the wire tip. Additionally, an angle α is determined from the curvature of the reconstructed guidewire (Fig. 2a). Adaption of the plane orientation is only performed when α is larger than a pre-defined minimum.
A vessel mask with definition of the envisioned intervention path can be used as a-priori information to align the real-time slices to the vascular system. Figure 3 shows, exemplarily for a vessel MRI phantom, the segmented vessel mask with a defined path for intervention (a). A smoothed representation of the path is computed and synchronized to the wire tip (b). The image plane calculation uses samples of the path representation that are (1) in front of the wire tip, (2) near to the wire tip and (3) behind the wire tip to align the new image plane with the vascular system (c-d).
The full workflow of the real-time scanner interaction is summarized in Figure 4. After retrieving the MRI images via ethernet, the guidewire is located and reconstructed before calculating the new image plane position and orientation. All image processing and calculation steps are performed on a separate computer and results are fed back to the scanner console. The real-time slice steering was tested in experiments using 3T MRI scanners (MAGNETOM Skyra, Siemens Healthineers) at two different sites based on vessel phantoms mimicking vessels with different complexity.
Results
In the experiments, the algorithms included in the wire tracking and reconstruction of the wire geometry together with the calculation of the new imaging plane had an average computation time below 200ms, ensuring real-time capability of the slice steering. Figure 5a shows the additional value of using the vascular information for slice steering by removing the jittering observed when only using the wire geometry (see differences in the blue circles). Figure 5b and 5c show samples of a time series of real-time images with wire motion and automatic slice steering enabled.Discussion
Experiments show the importance of using information of the vascular system for automatic slice steering in MRI interventions. Otherwise, the real-time slice can become unstable and is prone to tracking errors. With the additional requirement regarding the angle between the sample points, the slice steering becomes smoother. In situations where this angle is not large enough, the adaption of the slice position only is sufficient for following the device. Low-level ghosting artifacts appear in the images when moving the imaging plane. This has to be observed in further work. The images also show distinct wrap-around artifacts that did not disturb wire tracking. This could be handled by using stronger oversampling in PE direction. However, in the sequence design, we aimed for high temporal resolution first. The real-time slice steering was implemented using a scanner-specific real-time interface (see Fig. 5c) (Access-i, Siemens Healthineers). However, we also set up the same workflow using the vendor-independent MRI framework gammaSTAR that allows the same, complex real-time interactions in a scanner-agnostic scenario (see Fig. 5b) [6-7].Conclusion
We presented a fast, real-time slice steering workflow for endovascular interventions that simplifies navigation through the vascular system by detecting the position of endovascular devices and adjusting the MRI acquisition in real time to follow the device. Vascular information from intervention planning are used to predict the course of the vessels for ideal slice positioning. Ultimately, this workflow can increase precision of positioning during MR-guided interventions and reduce radiation in widely applied treatments.Acknowledgements
Funding from the Eurostars-2 joint programme with co-funding from the EU Horizon2020 research and innovation programme (Eurostars E! 11263 - SPECTRE)References
[1] Chambers. Occupational health risks in interventional cardiology. JACC 2015;8:628–30.[2] Picano et al. The radiation issue in cardiology: the time for action is now. Cardiovasc Ultrasound. BioMed Central 2011;9:35.
[3] Hoinkiss et al. Guidewire tracking based on passive MRI markers for MR-guided endovascular interventions. ESMRMB 2020;L01.19.
[4] Griswold et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). MRM 2002;47(6):1202–10.
[5] Feinberg et al. Halving MR imaging time by conjugation: demonstration at 3.5 kG. Radiology 1986;161(2):527–31.
[6] Cordes et al. Portable and platform‐independent MR pulse sequence programs. MRM 2019;86(4):1277-90.
[7] Hoinkiss et al. Event-based traversing of hierarchical sequences allows real-time execution and arbitrary looping in a scanner-independent MRI framework. ISMRM 2020;1043.
Figures
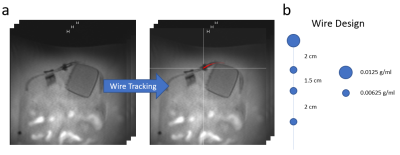
Figure 1: a)
Three parallel slices are acquired for 3D tracking of the guidewire which is
equipped with passive MRI markers that produce negative MRI artifacts. A wire
tracking algorithm detects the position of the MRI markers and reconstructs the
three-dimensional geometry of the guidewire. b) Marker position and
concentration (iron oxide nanoparticles) are shown for the wire used in the
experiments.
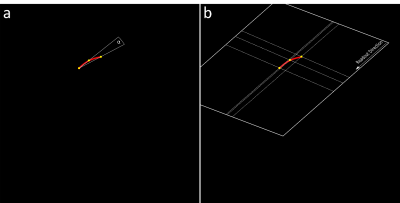
Figure 2: Imaging slice calculation based on the
reconstructed wire geometry. If the curve of the wire is larger than a
pre-defined angle, the orientation of the imaging plane is adjusted using the
plane equation and three sample points. Wire tip direction is then aligned with
the encoding axes of the acquisition.
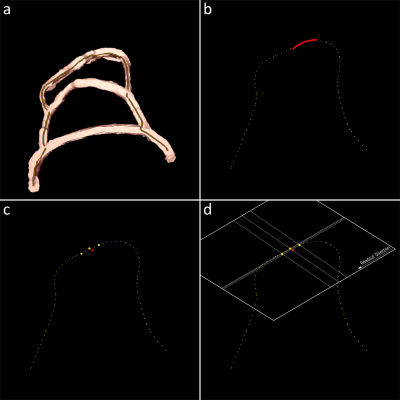
Figure 3: An
additional option allows to use a-priori information of pre-processed vessel
masks and the envisioned path for intervention (a). A smoothed path
representation is synchronized to the reconstructed wire geometry (b) and
sample points are calculated based on the interventional path (c) with which
the imaging plane information can be calculated (d). This allows to better
align the real-time MRI slices to the vascular characteristics.
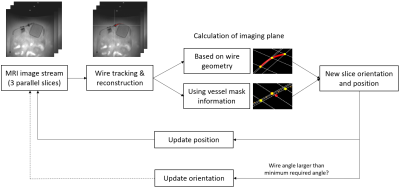
Figure 4: Overview
of the slice steering for endovascular interventions to follow endovascular devices
in real time. Wire reconstruction and the required imaging plane are calculated
on a separate computer. New slice orientation and position are sent back to the
MRI scanner for adjusting the imaging process.
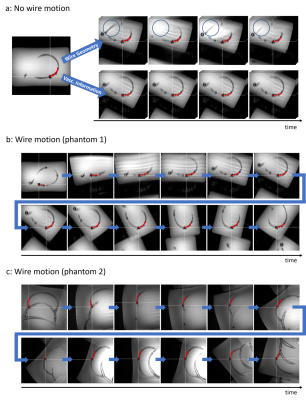
Figure 5: a)
Automatic slice steering based on reconstructed wire geometry (top) and
vascular information (bottom) with a non-moving wire. A slight jittering of the
real-time slices can be seen in the top row where orientation of the slices
changes between timepoints (see differences in blue circle). b) and c) Time
series of real-time images, acquired with slice steering and while performing guidewire
motion. The
experiments were performed at different MRI sites using two different MRI
phantoms.