0853
An MR Safe Steerable Catheter for MR-guided Endovascular Interventions1Hamlyn Centre for Robotic Surgery, Imperial College London, London, United Kingdom, 2Dept. of Radiology, Medical Physics, Medical Center, University of Freiburg, Freiburg, Germany, 3MaRVis Interventional GmBH, Krün, Germany, 4Shanghai Jiao Tong University, Shanghai, China
Synopsis
MR-guided intravascular interventions often lack steerable devices due to potential RF heating of long metallic components. In this study, we present a novel 7F MR safe and passively visible steerable catheter. Real-time MR image guidance of this catheter was demonstrated in a vessel phantom in a clinical 3T MR scanner to evaluate the catheter’s mechanical efficacy and MR visibility. Small vascular structures such as the renal artery could be probed efficiently with the polymer-only based catheter. With the integrated iron markers it was visible in its entirety, and the limited artifact size allowed for the effective visualization of surrounding tissue.
Introduction:
Minimally invasive surgery is increasingly gaining attention in interventional radiology and cardiology. X-ray fluoroscopy is currently the gold standard imaging technique for endovascular interventions, but it is associated with radiation exposure for both patients and medical personnel. MR-guided interventions do not use ionizing radiation, they provide anatomical and functional information and they allow for an early evaluation of therapy during the intervention1. However, MR-guided interventions still lack MR safe and compatible instruments, in particular steerable catheters. Steerability in catheters is often achieved with long metallic pull-wires, which can pose a safety hazard in the MRI environment due to unwanted RF heating. In this study, we present a prototype 7F steerable catheter with non-conducting pull wires that are passively visible, mechanically robust and fully MR safe.Materials and Methods:
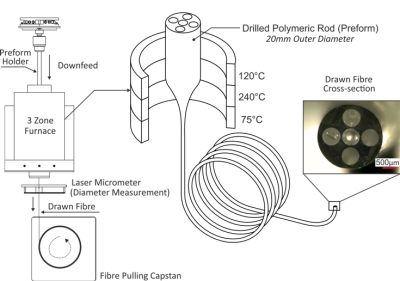
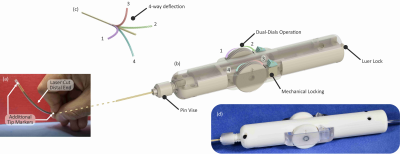
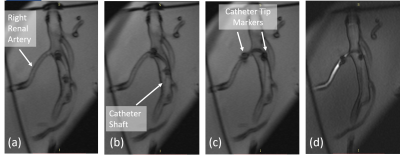
Steerable Catheter Fabrication: Using the relatively unconventional manufacturing method of fibre drawing2,3,4, a five-lumen polymeric tubing was fabricated to form the main building block of the 7F steerable catheter. From the tubing, (1) a flexible, yet relatively stiff shaft, and (2) a more flexible and a softer distal end materialize. Inspired by Clogenson et al.’s approach, the tubing was post-processed at its distal end using laser cutting to create the compliant steerable structure5. The flexible structure can be steered when pull-wires running from the proximal manual handle through the shaft towards the distal end are pulled. Unlike previous work where high strength synthetic fibres (Dyneema®5, Kevlar®6, Spectra®7) were utilized, the pull-wires herein are also passively MR visible over the full catheter length. The pull-wires employed are micropultruded rods used in the fabrication of MR safe MaRVis guidewires (MaRVis Interventional GmBH, Krün, Germany). These rods are thin composite materials, composed of non-metallic high strength synthetic fibres (glass and/or aramid fibres) impregnated by a high-temperature resistant epoxy resin. The epoxy resin is doped with iron microparticles as negative markers which cause susceptibility artefacts8. To achieve the multi-directional steerability, four pull-wires were incorporated in the four lateral (side) lumina of the tubing. To control the pull-wires, a handle with an ergonomic grip and two dials was 3D printed. At the distal end of the catheter, two additional passive markers (adhesive doped with iron microparticles) were added: one at the distal most point of the catheter and the other at the proximal end of the deflectable tip.Experiments: The steerable catheter was placed in a water-filled container for acquiring high resolution MR images in a clinical 3 Tesla MR system (Magnetom Prisma, Siemens) using a 3D FLASH sequence (TE/TR = 2.2/14.9ms, TA = 144.5s, α = 10°, FoV = 300*300mm2). Then, phantom experiments were performed under real-time MR image guidance to evaluate the mechanical efficacy and MR visibility of the steerable catheter. A soft silicone commercial vascular phantom of a normal adult abdominal aorta (Elastrat Sarl, Geneva, Switzerland) was filled with water, and the steerable catheter was introduced to target vascular structures such as the right renal artery. Real-time images were acquired using a 2D FLASH sequence with the following parameters: TE/TR = 2.2/319.6ms, TA/image = 320ms, α = 8°, FoV = 289*289mm2.
Results:
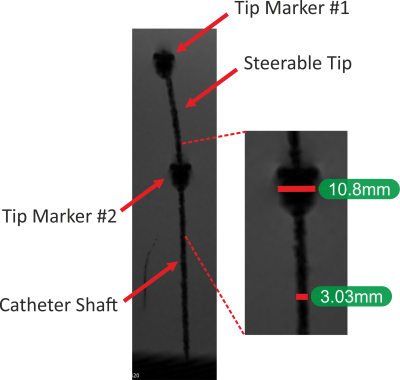
The five-lumen tubing was successfully fabricated using the preform to fibre manufacturing technique as shown in Figure 1. The cross-section shown in the figure (inset) demonstrates the successful scaling of the preform down to the desired tubing dimensions. The distal end of the tubing was post-processed (laser cut and Kevlar-braided) and coupled to the handle at the proximal end as shown in Figure 2. Figure 3 shows the acquired image from the 3D FLASH sequence and the size of the artifacts with a mean diameter of 3mm ± 0.2 along the shaft. Figure 4 shows the 2D Flash sequence of the successful probing by a novice of the right renal artery, which was followed by contrast agent injection for post-assessment.Discussion:
The fiber drawing technique to fabricate catheters allows for low-cost rapid prototyping which can lower the cost and accelerate research. While allowing miniaturization to desired dimensions, this approach still results in fabrication at long length scales for scalable manufacturing. The in-vitro experiments demonstrate that the polymer-only based catheter can be effectively manipulated in the vessel anatomy without obscuring the surrounding tissue. The size of artifacts is comparable to previous work9 with guidewires, which comprise an MR visible rod, as a continuous susceptibility artefact with a mean diameter of 2mm ± 0.2 along the shaft. Using 4 MR visible rods for steering did not increase the artifact size significantly.Acknowledgements
Grant support by the United Kingdom Engineering and Physical Sciences Research Council (EPSRC) (EP/P012779 Micro-Robotics for Surgery), and German Science Foundation (DFG) under grant number BO 3025/11-1 and CRC 1425 (Project P15); are gratefully acknowledgedReferences
1. Bock M & Wacker FK. MR-guided intravascular interventions: Techniques and applications. J. Magn. Reson. Imaging. 2008, 27: 326-338.
2. Temelkuran B, Hart SD, Benoit G, Joannopoulos JD, and Fink Y. Wavelength-Scalable Hollow Optical Fibres with Large Photonic Band Gaps for CO2 Laser transmission. Nature. 2002, 420: 650-653.
3. Abouraddy AF, Bayindir M, Benoit G, Hart SD, Kuriki K, Orf N, Shapira O, Sorin F, Temelkuran B & Fink Y. Towards multimaterial multifunctional fibres that see, hear, sense and communicate. Nature Materials. 2007, 6: 336-347.
4. Liu N, Abdelaziz MEMK, Gao A, Temelkuran B, Yang GZ, A Device, Patent Publication No: WO2020016577 (Published 23.01.2020).
5. Clogenson HCM, van Lith JY, Dankelman J, Melzer A & van den Dobbelsteen JJ. Multi-selective catheter for MR-guided endovascular interventions. Medical Engineering & Physics, 2015, 37: 623-630.
6. Bell JA, Saikus CE, Ratnayaka K, Wu V, Sonmez M, Faranesh AZ, Colyer JH, Lederman RJ & Kocaturk O. A deflectable guiding catheter for real‐time MRI‐guided interventions. J. Magn. Reson. Imaging. 2012, 35: 908-915.
7. Ataollahi A, Karim R, Fallah AS, Rhode K, Razavi R, Seneviratne LD, Schaeffter T & Althoefer K. Three-Degree-of-Freedom MR-Compatible Multisegment Cardiac Catheter Steering Mechanism. IEEE Transactions on Biomedical Engineering. 2016, 63: 2425-2435.
8. Heidt T, Reiss S, Krafft AJ, Özen AC, Lottner T, Hehrlein C, Galmbacher R, Kayser G, Hilgendorf I, Stachon P, Wolf D, Zirlik A, Düring K, Zehender M, Meckel S, von Elverfeldt D, Bode C, Bock M & von zur Mühlen C. Real-time magnetic resonance imaging - guided coronary intervention in a porcine model. Scientific Reports. 2019, 9, 8663.
9. Massmann A, Buecker A & Schneider GK. Glass-Fiber-based MR-safe Guidewire for MR Imaging-guided Endovascular Interventions: In Vitro and Preclinical in Vivo Feasibility Study. Radiology. 2017, 284: 541-551.
Figures