0852
3T-Chemical Shift Encoded MRI with Ultra-Short Echo Time Acquisition for Bone Quality Assessment: Preliminary Results in the Hip.1Radiology, NYU Langone Health, New york, NY, United States, 2Université de Lyon; CREATIS CNRS UMR 5220, Inserm U1206, INSA-Lyon, UCBL Lyon 1, Villeurbanne, France, 3Osteoporosis Center, Hospital for Joint Diseases, NYU Langone Health, New york, NY, United States
Synopsis
Osteoporosis (OP) is a disease associated with low bone mass and deterioration of bone microarchitecture leading to bone fragility and increased fracture risk, especially in the proximal femur. Therefore, we have developed a chemical-shift encoded acquisition performed with a spiral k-space sampling to acquire ultrashort echo-time and longer echo time in the echo train. This study aims to determine if uTE acquisition can be performed for fat/water separation and if additional information can be provided through cortical bone imaging.
Introduction
Osteoporosis (OP) is a disease associated with low bone mass and deterioration of bone microarchitecture leading to bone fragility and increased fracture risk, especially in the proximal femur. OP is a severe public health problem, with over 200 million worldwide suffering from this disease. The main imaging method to assess OP includes Dual Energy X-ray absorptiometry (DXA) for measuring areal bone mineral density (BMD). DXA is relatively easy to perform in the clinic and the amount of radiation exposure is low. Recent studies using MRI to assess bone quality and health have focused on bone marrow adipose tissue to assess its composition and quantity1,2. While early MRI methods predominantly exploited the signal arising from MAT through Chemical Shift Encoded (CSE) MRI, more recent approaches like ultrashort echo time (uTE) imaging enable more direct imaging of bone tissue and have only recently been applied notably to image the cortical bone in the spine and hip3-6. Usually, CSE-MRI is achieved with a Cartesian k space-filling multiple gradient echo acquisitions and cannot sample enough short echo time values to recover information about cortical bone, mainly composed of short T2 species. Therefore, we have developed a chemical-shift encoded acquisition performed with a spiral k-space sampling to acquire ultrashort echo-time and longer echo time in the echo train7,8. This study aims to determine if uTE acquisition can be performed for fat/water separation and if additional information can be provided through cortical bone imaging.Materials/methods
This study had institutional review board approval, and written informed consent was obtained from all subjects. MRI acquisitions were performed on n=5 subjects with osteoporosis (BMI= 19.5+/- 2.4 kg/m2; 48.41+/-7.7 years ) using a 3T system (Siemens Healthcare, Erlangen, Germany). First, we used a 3D spoiled gradient-echo sequence with an n=12 echoes train length (n ×1.2ms) with flyback readout gradient with the following parameters: TR/FA/NA = 16ms/5°/4 and BW= 2000 Hz/px; 32 coronal slices were acquired; Acquisition time =4 min. Then we acquired 2 interleaved 3D uTE stack of spirals with following parameters: TEs=0.08, 0.22, 2.4, 3.6, 4.8, 6.0, 7.2, 8.4, 9.6, 10.8, 12.0, 13.2ms; TR/FA: 16.2 ms / 5° ; BW= 975 Hz/px; Spiral duration= 800 µs ; Number of interleaved spirals= 800; Resolution= 1.4x1.4x1.7 mm3; SPIRIT reconstruction; Acquisition time =5min. Native time-series magnitude and phase images were saved from the scanner and processed using a homemade MATLAB script. We use the method previously described2 to compute Proton Density Fat Fraction (PDFF). For uTE acquisition, we used uTE echoes to compute porosity index9, weighted subtraction of long and short echo image10, and a short T2 component was fitted using a mono-exponential fit using the two first echo, (0.08 and 0.22 ms) assuming a similar long species contribution. For each reconstructed maps, the proximal femur was segmented and values integrated for the whole proximal femur, femoral head, femoral neck, and shaft. Linear regression between T-scores and uTE parameters was performed. PDFF values obtained using uTE and CSE were compared using Pearson's correlation coefficient.Results
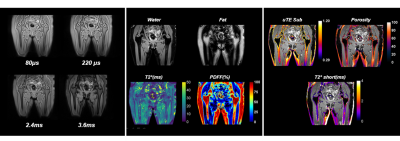
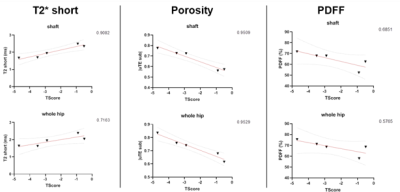
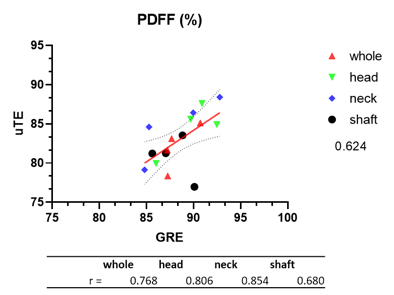
Typical acquired and reconstructed maps are shown in Fig.1. The regression plot between T-scores and uTE parameters are shown in Fig.2. Correlation between CSE and uTE PDFF is shown in Fig.3. We find that the T-score correlates with the uTE method's parameters in the whole hip bone and the shaft (Fig.2). T-score correlates with T2* short (r=0.71 in whole hip; r=0.90 in shaft), porosity index (r=0.95 in whole hip; r=0.95 in shaft) and PDFF (r=0.57 in whole hip; r=0.68 in shaft). We compared the PDFF value obtained with uTE with our standard CSE method and found good agreement between the two, notably in the femoral region (r=0.80 in femoral head and r=0.85 in femoral neck) (Fig.3)Conclusion
In this preliminary study, we demonstrate the feasibility of performing a single 3T uTE acquisition in vivo to assess metrics of bone health including T2*, cortical bone porosity, and marrow fat. The next step would be to compare the results in healthy vs. osteoporotic subjects.Acknowledgements
No acknowledgement found.References
1. Maas M, Akkerman EM, Venema HW, Stoker J, Den Heeten GJ. Dixon quantitative chemical shift MRI for bone marrow evaluation in the lumbar spine: a reproducibility study in healthy volunteers. J Comput Assist Tomogr 2001; 25(5): 691-7.
2. Martel D, Leporq B, Bruno M, Regatte RR, Honig S, Chang GJMri. Chemical shift-encoded MRI for assessment of bone marrow adipose tissue fat composition: pilot study in premenopausal versus postmenopausal women. 2018; 53: 148-55.
3. Chen M, Yuan H. Assessment of porosity index of the femoral neck and tibia by 3D ultra-short echo-time MRI. J Magn Reson Imaging 2018; 47(3): 820-8.
4. Guo Y, Chen Y, Zhang X, et al. Magnetic Susceptibility and Fat Content in the Lumbar Spine of Postmenopausal Women With Varying Bone Mineral Density. J Magn Reson Imaging 2019; 49(4): 1020-8.
5. Schmeel FC, Luetkens JA, Feisst A, et al. Quantitative evaluation of T2* relaxation times for the differentiation of acute benign and malignant vertebral body fractures. European journal of radiology 2018; 108: 59-65.
6. Sollmann N, Loffler MT, Kronthaler S, et al. MRI-Based Quantitative Osteoporosis Imaging at the Spine and Femur. J Magn Reson Imaging 2020.
7. Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution three-dimensional ultra-short echo time MR imaging. 2008; 60(1): 135-45.
8. Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). 2012; 67(2): 510-8.
9. Rajapakse CS, Bashoor-Zadeh M, Li C, Sun W, Wright AC, Wehrli FW. Volumetric Cortical Bone Porosity Assessment with MR Imaging: Validation and Clinical Feasibility. Radiology. 2015 Aug;276(2):526-35. doi: 10.1148/radiol.15141850. PMID: 26203710; PMCID: PMC4517853.
10. Du J, Bydder M, Takahashi AM, Carl M, Chung CB, Bydder GM. Short T2 contrast with three-dimensional ultrashort echo time imaging. Magn Reson Imaging. 2011;29(4):470-482. doi:10.1016/j.mri.2010.11.003
Figures