0851
Achieving Rapid and Accurate Relaxometry of Whole Knee Joint using Self-Supervised Deep Learning1Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States, 2New York University School of Medicine, New York, NY, United States, 3Orthopaedic Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
The purpose of this work was to develop and evaluate a model-guided self-supervised deep learning MRI reconstruction framework called REference-free LAtent map eXtraction (RELAX) for rapid quantitative relaxometry of the whole knee joint. This approach incorporated end-to-end CNN mapping to perform image-to-parameter domain transform. A concept of cyclic loss was utilized to enforce data fidelity and eliminate the explicit need for full-sampled training references. This approach was demonstrated in accelerated T1/T2 mapping of the whole knee joint and proven to outperform state-of-the-art reconstruction methods. The result suggests that RELAX allows accelerated relaxometry without training with reference data.
INTRODUCTION
Deep learning methods have been successfully used for image reconstruction with promising initial results. While these deep learning methods have focused on highly efficient image reconstruction for conventional MR imaging, applications for MR parameter mapping have been limited. A recent deep learning-based reconstruction framework, Model-Augmented Neural neTwork with Incoherent Sampling (MANTIS)[1], demonstrated an excellent capability of reconstructing quantitative maps directly from undersampled images using supervised learning. However, one major challenge of the standard supervised learning is the requirement of abundant training reference images, which can be difficult to acquire. The purpose of this study was to evaluate a general self-supervised deep learning reconstruction framework for quantitative MRI. This new technique, called REference-free LAtent map eXtraction (RELAX), jointly enforces data-driven and physics-driven training and use MR signal models in the network to guide self-supervised deep learning reconstruction. We demonstrated this technique on T1 and T2 mapping in knee MR imaging for rapid relaxation quantification of cartilage.METHODS
(a) RELAX Framework: The framework was shown in Figure 1. A CNN mapping (e.g. residual U-Net[2]) was implemented to convert the undersampled k-space data directly to the parameter maps through learning spatiotemporal information for end-to-end domain transform. The estimated latent parameter maps were provided into the known MR signal models to ensure a generation of synthetic MR data matching the acquired k-space data (i.e. model-guided data consistency for self-supervision). Unlike the standard supervised learning, this approach eliminated the need to guide CNN training with parameter maps obtained from fully-sampled k-space. Meanwhile, additional regularizations such as total variation (TV) on the latent parameter space can be applied to improve network training. The RELAX framework was evaluated for reconstructing T1 and T2 from undersampled k-space data in knee MRI. T1 mapping was performed based on variable flip angle (VFA) imaging with a spoiled gradient echo sequence [3], [4]. T2 mapping was performed based on multi-echo spin-echo (MESE) imaging [5]. The network was implemented using TensorFlow and trained on an NVIDIA GeForce RTX 2080Ti graphics card using adaptive gradient descent optimization with a learning rate of 0.0002 for 200 epochs.(b) Evaluation: The evaluation of T1 and T2 mapping was performed using two in-vivo knee image datasets. For VFA-based T1 mapping, images of the knee were acquired in the sagittal plane for 50 symptomatic patients using a 3T GE scanner (MR 750, GE Healthcare). Relevant imaging parameters included: TR/TE = 4.6/2.2 ms, 8 flip angle = [3, 4, 5, 6, 7, 9, 13, 18]°, slice thickness = 3mm, number of slices = 32, FOV = 16x16cm2, and image matrix = 256×256. For MESE T2 mapping, images were acquired in the sagittal plane for another 110 symptomatic patients using a 3T GE scanner (Signa Excite Hdx, GE Healthcare). Relevant imaging parameters included: 8 TEs/TR = [7, 16, 25, 34, 43, 52, 62, 71]/1500 ms, flip angle = 90°, slice thickness = 3-3.2mm, number of slices = 18-20, FOV = 16x16cm2, and image matrix = 320×256. A split of 80/20% of data was used to train/test the network. Retrospective undersampling was used with 1D variable density Cartesian patterns for an acceleration rate R=5. The reconstruction performance of RELAX was compared with k‐t SLR, representing the state-of-the-art low-rank and sparsity-based reconstruction approach [6], and MANTIS, representing the latest supervised learning quantitative mapping approach.
RESULTS
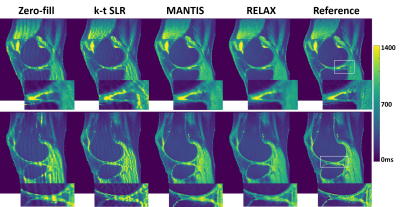
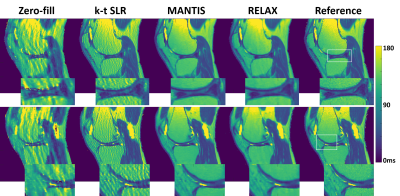
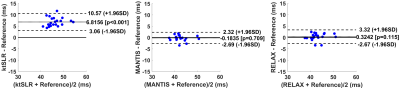
Figure 2 and 4 shows two slices of T1 and T2 maps for testing knee datasets at R=5. The deep learning-based methods, including both MANTIS and RELAX, removed most of the artifacts and showed a similar reconstruction performance, and outperformed the k-t SLR. The RELAX reconstruction generated T1 and T2 maps with image quality comparable to the reference maps. The absolute error map for each reconstructed T1 map was shown in Figure 3 at the bottom, which also indicates better reconstruction accuracy for both MANTIS and RELAX methods in comparison to conventional constrained reconstruction. Like Figure 3, the absolute error maps for T2 were shown in Figure 5, indicating better performance of MANTIS and RELAX. Figure 6 shows the Bland-Altman plots for the agreement of the mean cartilage T2 values of each subject (blue dot) in the testing knee datasets between the reference and the T2 maps reconstructed from different methods at an acceleration rate R=5. In contrast to k-t SLR, which typically overestimated T2 values, MANTIS and RELAX achieved unbiased estimation (p>0.05) of the T2 values for cartilage with narrower limits of agreements (the dashed lines).DISCUSSION
This work proposed a model-guided self-supervised deep learning reconstruction technique towards rapid quantitative MR parameter mapping. We have demonstrated that by enforcing MRI physical model constraints, deep learning-based MRI reconstruction may be performed without fully sampled training references. We tested the proof-of-concept for accelerated T1 or T2 mapping in knee MRI. Our initial results have supported that RELAX can generate T1 or T2 maps that are comparable to T1/T2 maps from standard supervised learning and superior to T1/T2 maps from the conventional constrained reconstruction. This new technique provides an emerging opportunity to accelerate quantitative MRI and holds great potential to improve accurate and reliable relaxometry in musculoskeletal imaging.Acknowledgements
No acknowledgement found.References
[1] F. Liu, L. Feng, and R. Kijowski, “MANTIS: Model-Augmented Neural neTwork with Incoherent k-space Sampling for efficient MR parameter mapping,” Magn. Reson. Med., Mar. 2019, doi: 10.1002/mrm.27707.
[2] O. Ronneberger, P. Fischer, and T. Brox, “U-Net: Convolutional Networks for Biomedical Image Segmentation,” in Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2015: 18th International Conference, Munich, Germany, October 5-9, 2015, Proceedings, Part III, N. Navab, J. Hornegger, W. M. Wells, and A. F. Frangi, Eds. Cham: Springer International Publishing, 2015, pp. 234–241.
[3] H. Z. Wang, S. J. Riederer, and J. N. Lee, “Optimizing the precision in T1 relaxation estimation using limited flip angles,” Magn. Reson. Med., vol. 5, no. 5, pp. 399–416, 1987, doi: 10.1002/mrm.1910050502.
[4] R. Venkatesan, W. Lin, and E. M. Haacke, “Accurate determination of spin-density and T1 in the presence of RF- field inhomogeneities and flip-angle miscalibration,” Magn. Reson. Med., vol. 40, no. 4, pp. 592–602, 1998, doi: 10.1002/mrm.1910400412.
[5] S. Meiboom and D. Gill, “Modified Spin‐Echo Method for Measuring Nuclear Relaxation Times,” Rev. Sci. Instrum., vol. 29, no. 8, pp. 688–691, Aug. 1958, doi: 10.1063/1.1716296.
[6] S. G. Lingala, Y. Hu, E. DiBella, and M. Jacob, “Accelerated Dynamic MRI Exploiting Sparsity and Low-Rank Structure: k-t SLR,” IEEE Trans. Med. Imaging, vol. 30, no. 5, pp. 1042–1054, May 2011, doi: 10.1109/TMI.2010.2100850.
Figures