0837
SPRING-RIO TSE: 2D T2-Weighted Turbo Spin-Echo Brain Imaging using SPiral RINGs with Retraced In/Out Trajectories1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Radiology & Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
This study presents a new approach to 2D TSE imaging using annular spiral rings with a retraced in/out trajectory, dubbed “SPRING-RIO TSE”, for fast T2-weighted brain imaging. Annular rings with retraced in/out segments offer benefits for reducing T2 decay induced artifacts and self-correction of moderate off-resonance effects. Phantom and human results show that high-quality T2-weighted images can be acquired with higher scan efficiency and reduced SAR, when compared with Cartesian TSE imaging.
Introduction
Spiral-based TSE techniques have shown advantages over conventional Cartesian TSE, in terms of SNR, CNR and reduced SAR1,2. Previously, we introduced a 2D SPRING TSE technique2 for dual-contrast T2-weighted images using spiral ring segmentation at 1.5T, and the results demonstrated that ring segmentation leads to a smoothed T2-dependent weighting of signal amplitudes across k space and thus to benign artifact behavior3,4. One key advantage of this annular ring segmentation, compared with an interleaved, rotated spiral-arm segmentation, is that there is no need for a double encoding strategy1 to mitigate the T2-decay induced signal variation, thus resulting in a shorter scan time. However, there are still challenges associated with this technique, such as residual T2-decay blurring, off-resonance induced signal loss, and relatively low SNR.In this work, a new technique combining a retraced in/out (RIO)5 acquisition with multi-shot spiral ring TSE is proposed to obtain T2-weighted brain images with sub-millimeter spatial resolution and improved image quality.
Methods
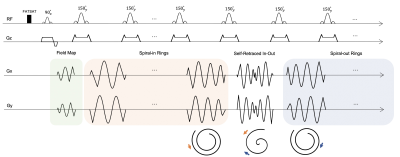
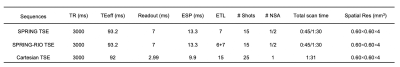
A schematic of the pulse sequence is depicted in Figure 1. A fat saturation pulse was used to suppress lipids before the TSE signal generation module. Short spiral-out arms were placed in the interval between the excitation and first refocusing sinc RF pulses for field-map estimation. The TSE data was then collected by a series of spiral rings, including a self-retraced spiral-in, spiral-out ring for the center of k space, and spiral-in rings at the beginning of the echo trains with corresponding retraced spiral-out rings at the end of echo trains for outer k space. Compared with the previous SPRING TSE implementation, this new RIO design has several benefits: (1) higher SNR due to the additional data acquired without scan time penalty yet still maintaining the desired TE; (2) reduced blurring artifacts induced by T2 decay; and (3) self-correction of the signal loss and artifacts caused by off-resonance signals.Two spiral-ring based TSE sequences (SPRING TSE, SPRING-RIO TSE) were implemented and tested. Spiral rings were designed by splitting the original constant density, multi-shot spiral-out arms into segments of equal time duration, each of which was played during one of the echo spacings in the TSE module. Model-based spiral trajectory correction6 was utilized for both sequences. Semi-automatic off-resonance correction with a minimum phase objective function7 was used for SPRING TSE, while a maximum energy objective function8 was used for SPRING-RIO TSE. Sequence parameters are given in Table 1.
Experiments were performed on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil array. For both phantom and healthy volunteer studies, multiple slices with 4 mm thickness and 2 mm gap were acquired using the proposed methods and Cartesian TSE as a reference.
Results and discussion
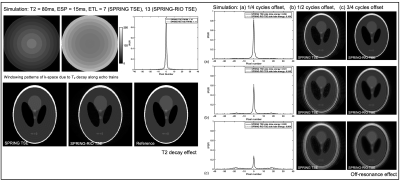
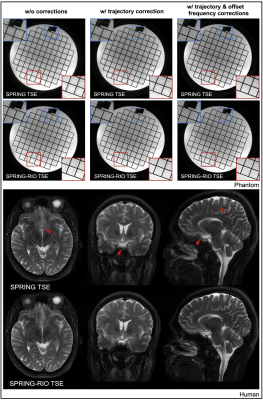
Simulation results in Figure 2 demonstrate the aforementioned benefits of using a RIO strategy in a spiral-ring TSE signal model. Figure 3a shows the performance of the corrections in a phantom study for both spiral-ring sequences. With trajectory correction, edge artifacts are reduced. By also performing off-resonance correction, both blurring artifacts and signal loss are significantly reduced. When compared with SPRING TSE, SPRING-RIO TSE is more sensitive to anisotropic gradient delays because of the trajectory retracing, while it is more robust to off-resonance effects via the self-correcting spiral k-space trajectory. The red arrows in Figure 3b, pointing to structures of the corrected brain images, show further evidence that SPRING-RIO TSE performs better than SPRING TSE with respect to overall image quality, including SNR, residual artifacts, and image blurring.Figure 4 shows a comparison between SPRING-RIO TSE with one signal average, and with two signal averages, and Cartesian TSE. The results indicate that the image quality of SPRING-RIO TSE with one signal average is, in general, comparable to that of Cartesian TSE, yet with only half of the scan time that was used for Cartesian TSE. With two signal averages, SPRING-RIO TSE shows a higher SNR and a similar or slightly better image contrast (white circles) than the Cartesian counterpart, even though its scan time is shorter. In terms of SAR, the refocusing RF angles are set to the same value used in Cartesian TSE, and the relative SAR from the refocusing RF pulses is approximately $$$(150^{2} \times 13)/(150^{2} \times 15) \approx 85\% $$$ of the SAR of Cartesian TSE.
Signal loss can still be seen in some regions where there are strong susceptibility gradients, and ghosting artifacts, likely induced by concomitant gradients, were observed in sagittal and coronal views, as shown in Figure 4 (red arrows). Future work will focus on more advanced off-resonance correction and concomitant gradient correction.
Conclusion
An improved 2D T2-weighted brain imaging using spiral-ring TSE was implemented and tested. The results show that the proposed method achieves 15-slice, high-quality T2-weighted images at 0.6 x 0.6 x 4 mm3 spatial resolution per slice with a high scan efficiency.Acknowledgements
No acknowledgement found.References
[1] Li Z, Karis JP, Pipe JG. A 2D spiral turbo-spin-echo technique. Magn Reson Med. 2018;80:1989–1996.
[2] Wang Z, Allen S, Feng X, Mugler JP, Meyer CH. SPRING TSE: 2D T2-Weighted Brain Imaging using SPiral RING Turbo Spin-Echo. Proceedings 28th Annual Meeting ISMRM, Virtual Conference. 2020:3714.
[3] Block W, Pauly J, Nishimura D. RARE spiral T2-weighted imaging. Magn Reson Med. 1997;37:582–590
[4] Hennig J, Menza M, Barghoorn A, Riemenschneider B, Kroboth S, Zaitsev M. Spiral RARE with annular segmentation. Proceedings 27th Annual Meeting ISMRM, Montreal. 2019:4632.
[5] Fielden SW, Meyer CH. A simple acquisition strategy to avoid off‐resonance blurring in spiral imaging with redundant spiral‐in/ out k‐space trajectories. Magn Reson Med. 2015;73:704–710.
[6] Tan H, Meyer CH. Estimation of k-space trajectories in spiral MRI. Magn Reson Med 2009;61:1396–1404.
[7] Chen W, Meyer CH. Semiautomatic off‐resonance correction in spiral imaging. Magn Reson Med. 2008;59:1212–1219.
[8] Allen SP, Feng X, Fielden SW, Meyer CH. Correcting image blur in spiral, retraced in/out (RIO) acquisitions using a maximized energy objective. Magn Reson Med. 2019;81:1806– 1817.
Figures