0834
Accelerated Ultrahigh Temporal-Resolution MRI with Random k-Space Undersampling1Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
It has been reported that a new sequence, epi-SPEEDI, can offer sub-millisecond temporal resolution, but its scan time is relatively long due to phase-encoding. In this study we propose an accelerated version of epi-SPEEDI (named epi-SPEEDI-kt) that improves the acquisition efficiency of epi-SPEEDI by randomly undersampling the k-space, followed by image reconstruction using joint spatiotemporal partial separability and sparsity constraints. Through a human imaging example for visualizing the dynamics of aortic valve, we demonstrated that epi-SPEEDI-kt can provide comparable image quality to epi-SPEEDI and reduce the scan time by ~50% concurrently. This acceleration is expected to enhance SPEEDI applications.
Introduction
MRI techniques capable of sub-millisecond temporal resolution began to emerge recently1-3, and one of these promising techniques is SPEEDI2. Despite its capability of ultrahigh temporal resolution, SPEEDI scans are relatively long due to the reliance on phase-encoding, even with a more efficient implementation – epi-SPEEDI3. This study aims at accelerating epi-SPEEDI acquisition by randomly undersampling k-space time series (termed epi-SPEEDI-kt), followed by image reconstruction based on joint spatiotemporal partial separability and sparsity constraints (PS-Sparse)4. We demonstrate the performance of epi-SPEEDI-kt technique through an example of visualizing the dynamic process of aortic valve opening/closing in humans with a temporal resolution of 0.6ms.Methods
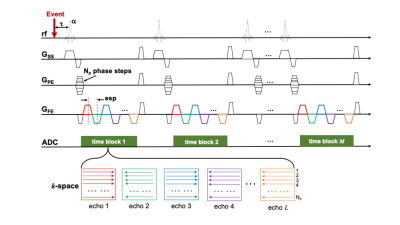
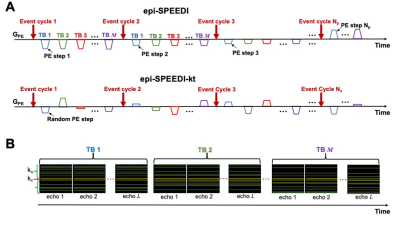
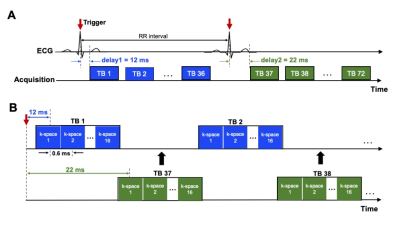
epi-SPEEDI-kt pulse sequence: the epi-SPEEDI-kt sequence is built upon epi-SPEEDI3 (Fig. 1) which utilizes an echo-train for readout without inter-echo blipped phase-encoding (PE) gradients. Each echo generates one separate k-space matrix corresponding to a distinct time delay relative to a trigger from a periodic event such as an ECG. Assuming echo-train length (ETL) = L and image matrix in PE direction = Np, L k-space matrices are filled after repeating the event and the echo-train acquisition Np times, and then these k-space matrices form a time block (TB) with the temporal resolution = echo spacing (esp). Typically multiple TBs are acquired to cover the entire periodic event. The epi-SPEEDI-kt pulse sequence differs from epi-SPEEDI in the PE scheme (Fig. 2A). Linear PEs are utilized in epi-SPEEDI and the PE step is the same across all the TBs during one event cycle, while epi-SPEEDI-kt employs random PE steps, which are different between TBs and event cycles. Furthermore, the acquisition is accelerated in epi-SPEEDI-kt by reducing the number of PEs to Ns (< Np), i.e., acceleration factor = Np/Ns. A fixed number (Nnav) of phase lines are sampled in the central k-space (kc) ((Np-Nnav)/2+1 to (Np+Nnav)/2) in all the TBs, and the other (Ns-Nnav) acquired phase lines are randomly and sparsely distributed in the outer k-space regions (ko) (Fig. 2B).Image acquisition: Images of the aortic valve were acquired from healthy subjects on a 3T GE MR750 scanner (General Electric Healthcare, Waukesha, WI) with a 32-channel phased-array cardiac coil. The scans were performed with epi-SPEEDI and epi-SPEEDI-kt using the following parameters: TR/TE = 20/8.8 ms, flip angle = 10º, FOV = 24 cm × 24 cm, slice thickness = 8 mm, ETL = 16, esp = 0.6 ms, matrix size = 80 × 118 (Np = 80) for epi-SPEEDI and Ns/Nnav = 40/6 in epi-SPEEDI-kt. To fill in the time gaps between TBs, an “interleaved multi-phase” acquisition scheme was adopted with two ECG trigger delays (12 and 22 ms) and 36 cardiac phases per delay (Fig. 3). The epi-SPEEDI/epi-SPEEDI-kt scans were completed with 160/80 heart beats and 8/4 breath-holds. The total scan time was about 6/3 minutes including the preparation time between breath-holds, which represented a two-fold scan time reduction with epi-SPEEDI-kt.
Image reconstruction: A total of 1152 k-space matrices (72 TBs x ETL) were acquired from each scan and reconstructed offline using customized MATLAB programs (MathWorks, Inc., Natick, MA). The epi-SPEEDI data were reconstructed from each coil channel with FFT followed by a sum-of-squares combination. To reconstruct the epi-SPEEDI-kt images, the k-space matrices at the same echo index were extracted from all the TBs to form 16 echo k-space time series, each of which included 72 time points. The PS-Sparse algorithm4 was then applied to reconstruct individual echo k-space time series. Finally, the reconstructed images were aligned in the time order relative to the ECG trigger.
Results
As shown in Fig. 4, the rapid opening and closing process of aortic valve was observed in both the epi-SPEEDI (Fig. 4A) and epi-SPEEDI-kt (Fig. 4B) scans with a temporal resolution of 0.6 ms. Compared to epi-SPEEDI, epi-SPEEDI-kt provided comparable image quality for visualizing the aortic valve opening and closing, despite a two-fold scan time reduction. Furthermore, slight artifacts were observed in lower regions of some images (outside the heart) in the epi-SPEEDI time series (Fig. 4A), but not in epi-SPEEDI-kt. These artifacts could be caused by inconsistent respiration positions across multiple breath-holds. This observation implies that the reduced times of breath-holds afforded by epi-SPEEDI-kt likely decreased the susceptibility to breath-holding inconsistency and thus improved the image quality.Conclusion and Discussion
Our study demonstrated that the epi-SPEEDI-kt technique was able to reduce the scan time of epi-SPEEDI by approximately 50% and offered comparable image quality in imaging the dynamics of aortic valve opening/closing. Further, epi-SPEEDI-kt required fewer breath-holds, which improves not only the reliability of SPEEDI imaging, but also patient comfort and compliance. In this study, although the feasibility of epi-SPEEDI-kt was illustrated with a cardiac imaging study, the technique is expandable to other applications for capturing ultrafast dynamic events. In addition, The PS-Sparse reconstruction method would achieve better performance with a larger number of time points4, enabling higher accelerations beyond what was investigated in this study. In summary, epi-SPEEDI-kt is a viable way to implement SPEEDI techniques. By reducing the scan time, epi-SPEEDI-kt has provided an enhanced tool for studying ultrafast, periodic physiological and physical processes with a sub-millisecond temporal resolution.Acknowledgements
This work was supported in part by the National Institutes of Health (1S10RR028898-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are grateful to Dr. Muge Karaman and Guangyu Dan for helpful discussions.References
1. Fischer J, Abels T, Özen AC, Echternach M, Richter B, Bock M. Magnetic resonance imaging of the vocal fold oscillations with sub‐millisecond temporal resolution. Magn Reson Med 2020;83:403–411.
2. Zhong Z, Sun K, Karaman MM, Zhou XJ. MRI with Sub-Millisecond Temporal Resolution. Magn Reson Med. 2020; 00:1–11. doi: 10.1002/mrm.28588.
3. Zhong Z, Sun K, Dan G, Karaman MM, Zhou XJ. Capture the Opening and Closing of Human Aortic Valve Using MRI with Sub-Millisecond Temporal Resolution. In: Proc Intl Soc Magn Reson Med Virtual Conference; 2020. P1198.
4. Zhao B, Haldar JP, Christodoulou AG, Liang Z-P. Image reconstruction from highly undersampled (k, t)-space data with joint partial separability and sparsity constraints. IEEE Trans Med Imaging 2012;31:1809-1820.
Figures