0832
Mesenchymal Stem Cell Impacts on Cerebral Microstructural Diffusion Recovery After Ischemic Attack1National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, FAMU-FSU College of Engineering, Tallahassee, FL, United States
Synopsis
DTI, NODDI and DKI techniques were applied to diffusion data acquired at 21.1 T to identify microtissue changes in ischemic brain tissue following adult human mesenchymal stem cell (hMSC) or control treatment. Scanning was conducted 1-21 d post-MCAO and treatment. 2D hMSC significantly reduced cell swelling (ICVF) at nearly every time-point in white matter while preserving orientation integrity (ODI), normal levels of DTI metrics, and early phase kurtosis. In grey matter, 2D hMSC restored DTI metrics to naïve levels quicker than control treatments, as did ICVF and ODI. Kurtosis had high variability, with minor trends evident.
Introduction
Stroke occurrence is a widely researched topic in the context of diffusion MRI. DTI quantifications of mean, axial and radial diffusivity (MD, RD and AD, respectively) as well as fractional anisotropy1 provide insights into hindered diffusion. As DTI protocols are not effective probes of non-Gaussian water displacement associated with restricted diffusion, other probes of tissue microstructure like DKI2 can evaluate intra-voxel diffusion tortuosity. Further, NODDI3 assesses intra-, extra-cellular and CSF compartments, and neuronal orientation. Though initially utilized to quantify neurite density, the intracellular volume fraction (ICVF) should provide an indicator of neurite swelling in the context of stroke pathology with the potential for the orientation dispersion index (ODI) to be impacted as well. Studies have shown the therapeutic efficacy of adult human mesenchymal stem cells (hMSC) in treating ischemic stroke4,5, but none have compared the aforementioned diffusion MRI techniques in characterizing them. This study aims to compare DTI, NODDI and DKI metrics to evaluate ischemic recovery following hMSC treatment.Methods
Animal and Cell Model: Male Sprague Dawley rats (220-250 g) underwent 1-h transient middle cerebral arterial occlusion (MCAO) followed by immediate IA injection of 1 mil. 2D hMSC (n=5) or physiological phosphate buffered saline (PBS) as control (n=6). Prior to injection, hMSC were incubated with 7.47-µg/mL micron-sized particles of iron oxide (MPIO) for 12 h for visualizing the initial biodistribution of implanted stem cells.MR Techniques: Using the 21.1-T ultra-widebore magnet at the US National High Magnetic Field Laboratory and a linear 1H/23Na birdcage coil, high resolution images were acquired at days 1-21 post-MCAO/injection. Diffusion EPI was acquired at b values 1 ms/μm2 (20 dir), 2 ms/μm2 (20 dir) and 3 ms/μm2 (30 dir): TE/TR=23.5/4000 ms, resolution=200x200x1000 μm, 31 slices, and scan time of 78 min, plus respiratory trigger. hMSC localization was performed on day 1 of imaging and required a standard 2D FLASH sequence: TE/TR=4/1000 ms, resolution=50x50x500 μm, 31 slices, and acquisition time of 10 min.
Data Analysis: DTI maps were processed with DiPy6. NODDI3 and Kurtosis7 processing were conducted in MATLAB. Resultant maps from day 1-21 were segmented with ROI of the external capsule and striatum to assess longitudinal changes and group differences. Statistical analysis (JMP, SAS Inc) was performed using a Mixed Model (p<0.05) and Tukey’s HSD post-hoc test.
Results
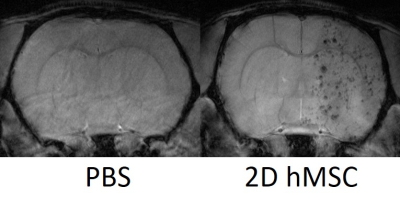
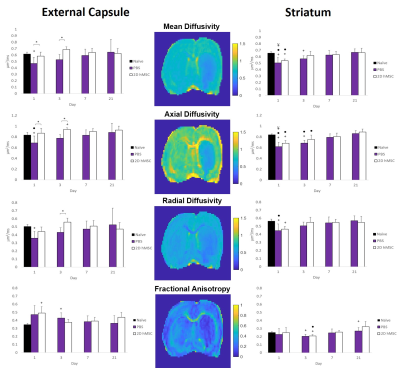
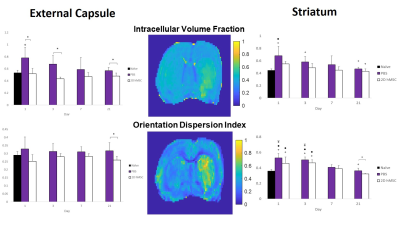
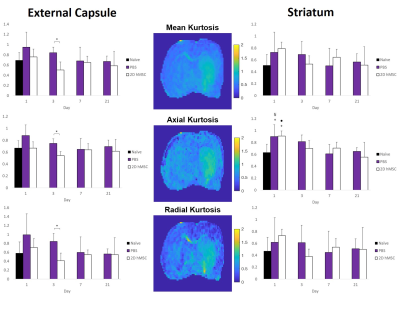
Successful IA delivery of hMSC were confirmed via 2D FLASH imaging (Fig. 1).In the ipsilateral external capsule: MCAO PBS-injected controls had significantly lower MD and AD (Fig. 2) on days 1 and 3, as well as RD on day 3, relative to the hMSC cohort. The PBS MD and RD were significantly lower than naïve on day 1. ICVF and ODI (Fig. 3) were nearly identical between naïve and hMSC groups, while PBS animals had significantly elevated ICVF at nearly every time point. Kurtosis (Fig. 4) showed differences mainly on days 1 and 3, with hMSC-treated animals having a much lower kurtosis in each direction.
In the ipsilateral striatum: PBS and hMSC-treated animals displayed near identical patterns, but for the DTI metrics, the hMSC group reached naïve levels quicker than PBS. Control and treatment showed similar trends in recovery of ICVF and ODI, though the hMSC group appears to have, on average, less dramatic shifts compared to naïve. PBS ODI did not recover to normal levels by the final time point. Kurtosis had high variability for all groups, with reduced variability in the axial direction. Both PBS and hMSC groups had significantly higher AK than naïve.
Discussion & Conclusion
In the external capsule, 2D hMSC display clear preservation of ICVF as a marker of cellular swelling, with reduced restriction and tortuosity at early time points comparable to PBS. DTI and DKI metrics have similar trends in this region, indicating that the path of diffusion is likely impacted uniformly by restrictions without directional specificity. Orientation and white matter integrity (ODI) are not significantly impacted by these ischemic events, with or without treatment. Markedly lower kurtosis in the hMSC group on day 3 and slightly lower on day 1 imply that hMSC reduce tortuosity in the acute phase within the external capsule. It is unclear why ICVF in PBS animals is significantly higher than the hMSC group at day 21, considering the restoration and comparability of DTI and DKI variables between groups observed after day 3. Longitudinal changes are not large due to standard deviations, but trends are apparent.In the striatum, differences between PBS and treatment groups are absent. Mean and axial diffusivity for the hMSC group are more comparable to naïve measures by day 3. ICVF is on average higher in the control group, most notably compared to naïve at day 1 and 3. However, striatal ODI seems to recover only for the hMSC group, highlighting the restorative capabilities of stem cell intervention. Similar trends between ODI and DTI measures could mean that grey matter restriction is the result of structural impairments post-MCAO. Though with high variability, AK displayed the lowest variances and some consistency over time, returning towards naïve levels for all groups by day 21. RK, and by extension MK, appear more variable for the hMSC group, which may have physiological implications longitudinally, but require further investigation.
Acknowledgements
This work was supported by the NIH (RO1-NS102395). The National High Magnetic Field Laboratory is funded by the NSF (DMR-1644779) and the State of Florida.References
1. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson - Ser B. 1996;111(3):209–19.
2. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432–40.
3. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–16.
4. Yuan X, Rosenberg JT, Liu Y, Grant SC, Ma T. Aggregation of human mesenchymal stem cells enhances survival and efficacy in stroke treatment. Cytotherapy. 2019;21(10):1033–48.
5. Helsper S, Bagdasarian FA, Yuan X, Xu K, Lee J-Y, Rosenberg JT, et al. Implantation of Human Mesenchymal Stem Cells Indicates Ischemic Recovery Assessed by Sodium MRI at 21.1 T. Transl Stroke Res. 2020;In Review.
6. Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 2014;8:8.
7. Veraart J, Poot DHJ, Van Hecke W, Blockx I, Van der Linden A, Verhoye M, et al. More accurate estimation of diffusion tensor parameters using diffusion Kurtosis imaging. Magn Reson Med. 2011;65(1):138–45.
Figures