0831
Mechanisms of cerebral ischemic stroke recovery from stem cell derived therapies assessed via MRI at 21.1 T1National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, FAMU-FSU College of Engineering, Tallahassee, FL, United States
Synopsis
This study evaluates therapeutic efficacy of human mesenchymal stem cell (hMSC) derived treatments applied to an ischemic stroke rat model. The goal is to determine if the presence of hMSC or hMSC derivatives in an ischemic region is required or if delivery of cell secretions alone can improve outcomes, either locally at the lesion or by recruiting regenerative neural progenitor cells. Biochemical markers of tissue recovery measured longitudinally using sodium chemical shift imaging and relaxation-enhanced MR spectroscopy at 21.1 T enables increased sensitivity, enabling insight into ionic and metabolic regulation while enabling to determine therapy efficacy.
Introduction
Trophic and immunomodulatory effects render human mesenchymal stem cell (hMSC) promising candidates for cell therapy in stroke treatment. We have demonstrated MRI to be a sensitive metric to assess therapy efficacy in an ischemic stroke model via biochemical markers measured longitudinally using sodium (23Na) chemical shift imaging (CSI) and relaxation enhanced MR spectroscopy (RE-MRS). Ultra-high field at 21.1 T provides increased sensitivity, enabling insight into ionic and metabolic homeostasis while assessing therapeutic efficacy. This approach may yield a particularly advantageous method to investigate hMSC derivatives such as extracellular vesicles (EV), which are endosomally-produced vesicles that carry immunomodulatory and regulatory secretions.1,2 EV should target neurodegeneration,3 and appear to aid in tissue recovery following ischemic stroke in rat models.3–5 Similar to cellular therapy, in vivo tracking of EV is imperative to determining bio-distribution, which has remained limited in MRI applications. Labeling of EV with superparamagnetic iron oxides (SPIO) contrast agents via sonication is proposed.Methods
Cell Culture: hMSC were acquired from Tulane Center for Stem Cell Research and Regenerative Medicine and characterized based on plastic adherence and surface markers.6 Evaluations of hMSC included population-doubling time, colony forming ability, immunomodulation and replicative senescence. For in vivo transplantation, hMSC were exposed to 7.47-µg Fe/mL micron-sized particles of iron oxide for 12 h and washed just prior to injection.EV Preparation: EV were isolated from cell culture, exposed to 0.5-1 mg/mL of SPIO nanoparticles under sonication and purified via centrifugation. T1, T2 and T2* relaxation measurements were completed on labeled EV embedded in 1% agarose gel. EV will be injected intra-arterially for direct comparisons to cellular injections.
Animal Model: A transient middle cerebral artery occlusion model7 was instituted in Sprague-Dawley rats (200–250 g) for 1 h, inducing striatal ischemia, followed by immediate arterial injection of the hMSC-derived therapy (cells or EV). Behavioral characterization was conducted concurrently with MR scanning to 21-d post-ischemia.
MR Acquisitions: Data were acquired using the 21.1-T, 900-MHz vertical magnet at the NHMFL. In vitro assessment of SPIO labeling of hMSC-EV utilized a 10-mm 1H radiofrequency coil. In vivo assessment utilized a linear birdcage double-tuned 23Na/1H radio frequency coil on 1, 3, 7 and 21 d post-ischemia to assess tissue recovery and treatment efficacy. Cell administration was confirmed with gradient recalled echo images (50x50-µm, in-plane resolution). 3D 23Na CSI was acquired at 1-mm isotropic resolution. RE-MRS8 evaluated metabolites in both ischemic and contralateral hemispheres. Selective bandwidth excitation pulses targeted lactate, creatine, choline and N-acetyl aspartate, while avoiding water, with spatial selectivity imparted by adiabatic selective refocusing (LASER)8 pulses. T2W images enabled anatomical reference to the ischemic lesion and contralateral alignment.
Analysis: EV labeling efficiency was verified via signal analysis with corresponding relaxation curves and rates generated in SigmaPlot 11.0 (Systat Software, Inc.). CSI data reconstructed in MATLAB was zero-filled to 0.5-mm isotropic resolution for volumetric and signal analysis in Amira 3D Visualization Software (Thermo Fisher Scientific, Inc). A signal threshold generated from the contralateral hemisphere was used to define the ischemic lesion. Peaks for RE-MRS data was assigned according to previous literature (NAA 2.0 ppm, Lac 1.31 ppm, Cre 3.0 ppm, Cho 3.2 ppm),9 referencing water at 4.7 ppm. Metabolite ratios to choline were used to track metabolic signals longitudinally. Statistical significance is shown according to a Student’s T-test and all graphs are mean ± SD.
Results
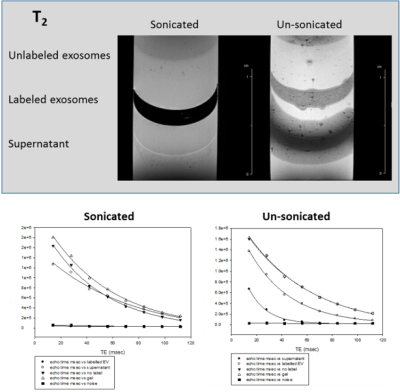
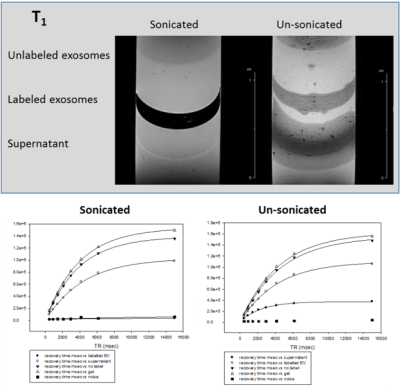
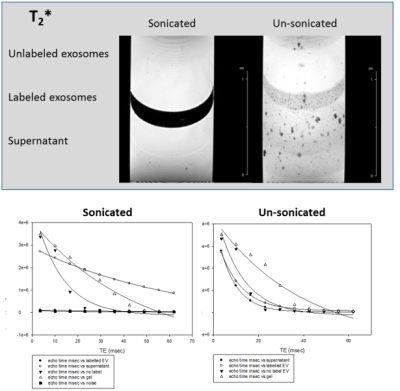
MRI of EV labeled with SPIO via sonication to achieve internalization is demonstrated in Figs.1-3 with comparison to unlabeled samples as control and SPIO-exposed EV without sonication. Increased SPIO uptake was achieved under sonication compared to un-sonicated samples as confirmed through signal measurements in both the EV and supernatant layers.Preliminary results indicate hMSC-treated cerebral ischemia reduces ischemic lesion by 19.8% compared to an increase of 15.6% in volume for control animals by day 3 when assessed via 23Na CSI (Fig.4). 23Na signal changes parallel this trend with a 10.8% signal recovery compared to 34.6% signal increase, respectively. Furthermore, approximately 25% increase in Lac/Cho levels is expressed in control animals compared to hMSC-treated.
Discussion
T1, T2 and T2* relaxation parameters suggest optimal uptake of SPIO contrast agent in EV. Preliminary results will extend to in vivo assessment of hMSC-EV to determine bio-distribution following administration and efficacy compared to cellular therapy. hMSC-treated animals have demonstrated increased recovery from cerebral ischemia compared to control animals. In vivo comparison of cell and EV administered therapy will provide insight into the mechanisms of recovery instituted by hMSC, either through direct cell interaction with the local environment or secretions alone. Previous studies have demonstrated 23Na MRI and 1H RE-MRS are sensitive biomarkers of therapy efficacy, and have been able to differentiate successful from ineffective therapies as early as 1 d post-injection.Conclusion
Determining the efficacy of hMSC-derived therapy using MR is a feasible approach to provide valuable feedback in assessing mechanism of recovery in cerebral ischemia. Labeling of hMSC-EV with MRI contrast to aid in biodistribution properties is proposed.Acknowledgements
All work has been conducted in accordance with the Florida State University Animal Care and Use Committee. This work was supported by the US NIH (RO1-NS102395 awarded to S.C. Grant and F31 NS115490 awarded to S. Helsper). The National High Magnetic Field Laboratory is funded by the US NSF (DMR-1644779) and the State of Florida.References
1. Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
2. Vlassov, A. V. et al. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta - Gen. Subj. 1820, 940–948 (2012).
3. Perets, N. et al. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 19, 3422–3431 (2019).
4. Xin, H. et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–5 (2013).
5. Busato, A. et al. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int. J. Nanomedicine 11, 2481–90 (2016).
6. Yuan, X. et al. Aggregation Preconditioning Enhances Survival and Efficacy of Human Mesenchymal Stem Cells in Stroke Treatment. Cytotherapy 21, 1033–1048 (2019).
7. Longa, E. Z., et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989).
8. Shemesh, N. et al. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nat. Commun. 5, (2014).
9. Govindaraju, V. et al. A. Proton NMR chemical shifts and coupling constants for brain metabolites. (2000).
Figures