0829
Recanalization of Acute Intracranial Large Vessel Occlusions: Novel Findings from High-Resolution Vessel Wall Imaging1Department of Radiology, First Central Clinical College, Tianjin Medical University, Tianjin, China, 2Department of Radiology, Tianjin Huanhu Hospital, Tianjin, China, 3Department of Radiology, Tianjin Medical University Nankai Hospital, Tianjin, China, 4MR Collaboration, Siemens Healthcare Ltd., Beijing, China, 5Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin, China, 6Department of Radiology, Tianjin First Central Hospital, School of Medicine, Nankai University, Tianjin, China
Synopsis
Pre-operative recanalization assessment for acute intracranial large-vessel occlusion (LVO) can help optimize endovascular therapy and shorten procedural times. We prospectively examined 46 patients with acute intracranial LVO who underwent high-resolution magnetic resonance imaging (HRMRI) before endovascular therapy. HRMRI had good agreement with angiographic assessment of the causes of occlusion (κ=0.89, 95% CI, 0.69–1.00) and length of occlusion (concordance correlation coefficient=0.75, 95% CI, 0.59–0.86). Intraluminal enhancement was associated with procedural complexity (r=0.81, P< .001) and procedural times (r=0.64, P< .001). HRMRI before recanalization can help define the vascular status and assist with endovascular therapy of acute intracranial LVOs.
Introduction
Preoperative assessment for recanalization of acute intracranial large-vessel occlusion (LVO) is important for optimizing endovascular therapy and shortening procedural times.1 We investigated the pre-recanalization vessel characteristics, including occlusion cause and lengths of LVO based on HRMRI,2 and relationships between the pre-surgical diagnostic imaging characteristics and the vessel characteristics found during the surgical procedures.Methods
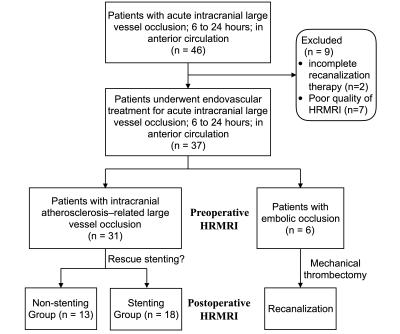
We prospectively recruited 46 consecutive patients with acute intracranial LVOs who received endovascular treatment. Subjects met the inclusion criteria of3 (1) acute ischemic stroke within 6-24 h of symptom onset; (2) pre-treatment occlusive lesions in the middle cerebral and distal internal carotid arteries; (3) endovascular treatment performed; and (4) HRMRI before endovascular therapy. The exclusion criteria were (1) poor HRMRI quality and (2) cessation of endovascular treatment because of poor medical condition.Rescue endovascular treatment (intra-arterial tirofiban infusion, balloon angioplasty, or intracranial stenting4 ) was performed when the recanalized occluded segment had significant stenosis.1 A stepwise approach for using these modalities, in addition to mechanical thrombectomy, were used to achieve successful recanalization.
HRMRI sequences were acquired on a 3T MR imaging scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 64-channel integrated head/neck coil. HRMRI was acquired with a pre-contrast and post-contrast 3-dimensional turbo spin-echo technique known as T1w–Sampling Perfection, with application-optimized contrasts using different flip-angle evolution (SPACE). The parameters were repetition time=900 ms; echo time=15 ms; field of view=200 × 200 mm; matrix=320×320; number of slices=224; slice thickness=0.53 mm; voxel size=0.6×0.6×0.5 mm3; acquisition time=7 min. Post-contrast HRMRI was performed five minutes after the injection of a gadolinium-based contrast agent (Magnevist; Schering, Berlin, Germany).
The causes of the occlusions were identified, based on the occlusion type: Branching-site or truncal-type occlusions were considered embolic occlusion or acute intracranial atherosclerosis-related LVO (ICAS-LVO), respectively.5 The occluded segment was defined as an embolus or ICAS site with a thrombus, which had focally uneven vessel walls with intraluminal heterogeneous enhancements. Slow flow was defined as smooth vessel walls with intraluminal homogeneous enhancements. Intraluminal enhancements of the occluded segment were graded on a 3-point scale: grade 0, none or signal equal to that of the pre-contrast image; grade 1, weak enhancement with a signal greater than that of the pre-contrast image, but less than that of the pituitary stalk; grade 2, strong enhancement with a signal greater than that of the pre-contrast image, and equal to or greater than that of the pituitary stalk.6 All patients underwent HRMRI scans three days after endovascular therapy.
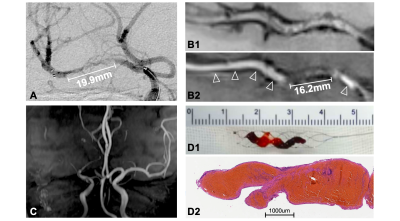
The occluded and slow-flow segments were measured on angiographic images and used as the reference standard. The ICAS-LVO was defined as a significantly fixed focal stenosis at the occlusion site. An occlusion was considered embolic if the vessel at the occlusion site was smooth and the vessel was completely recanalized after thrombectomy. Slow flow was defined as low-velocity flow in an artery or retrograde filling of a branch artery via leptomeningeal collaterals when an arterial occlusion was present. Occluded segment lengths were measured when a stent retriever was deployed.
Results
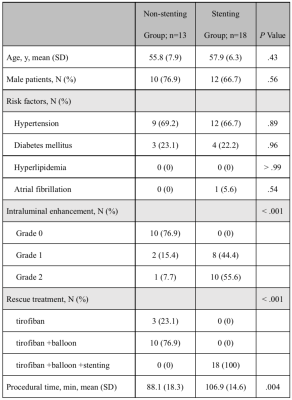
Thirty-seven patients met the eligibility criteria (Figure 1). HRMRI and angiographic assessments were in good agreement for measurement of the occluded segment (concordance correlation coefficient=0.75, 95% CI, 0.59–0.86, Figure 2). Of 37 patients, 31 had ICAS-LVOs in the truncal site, and slow flow was visible in all cases. Six patients had embolic occlusions in the branching site. Agreement was high between the HRMRI and angiographic assessments for identification of the causes of occlusion (κ=0.89, 95% CI, 0.69–1.00). Of the 31 patients with ICAS-LVOs, 18 received rescue stenting, and 13 received no further treatments. The stenting group had higher intraluminal enhancement grades than did the non-stenting group (P< .001, Table 1). Procedural complexities (r=0.81, P< .001) and procedural times (r=0.64, P< .001) were positively correlated with the intraluminal enhancement grades.Discussion
HRMRI revealed occlusion sites and differentiated the causes of occlusion. In the narrow spectrum of LVO causes, truncal-type occlusions might be derived from in situ thrombo-occlusions caused by underlying ICAS lesions. Liu et al.7 reported that enhancements could be a marker of atherosclerotic plaques. However, in situ thrombi usually do not have enhancements because of the lack of a nutrient artery. Therefore, higher grade enhancements might indicate that, in occluded segments, atherosclerotic plaque percentages are higher than those of situ thrombi (Figure 3). The histologic content of occluded segments has been reported to influence mechanical characteristics of occlusion, which can affect the difficulty of recanalization.8 In this study, the lengths of occluded segments were accurately estimated with HRMRI before recanalization procedures were performed. HRMRI could help identify the most distal and proximal boundaries of occluded segment so that the entire length of an occluded segment could be covered with balloons or stents to improve recanalization success rates.9Conclusions
HRMRI was comparable to angiographic assessment in determining the cause and length of vessel occlusion in patients with acute intracranial LVO. The use of HRMRI in determining the appropriate treatment for LVO might help shorten procedural times and provide better clinical outcomes.Acknowledgements
This study was supported, in part, by the Natural Science Foundation of China (NSFC) (81871342) and, in part, by the National Key R&D Program of China (2019YFC0120903).References
- Baek J, Kim B. Angiographical identification of intracranial, atherosclerosis-related, large vessel occlusion in endovascular treatment. Frontiers in neurology. 2019;10:298
- Arenillas J, Dieleman N, Bos D. Intracranial arterial wall imaging: Techniques, clinical applicability, and future perspectives. International journal of stroke : official journal of the International Stroke Society. 2019;14:564-573
- Albers G, Marks M, Lansberg M. Thrombectomy for stroke with selection by perfusion imaging. The New England journal of medicine. 2018;378:1849-1850
- Chang Y, Kim B, Bang O, Baek J, Heo J, Nam H, et al. Rescue stenting for failed mechanical thrombectomy in acute ischemic stroke: A multicenter experience. Stroke. 2018;49:958-964
- Baek J, Kim B, Heo J, Kim D, Nam H, Kim Y. Outcomes of endovascular treatment for acute intracranial atherosclerosis-related large vessel occlusion. Stroke. 2018;49:2699-2705
- Al-Smadi A, Abdalla R, Elmokadem A, Shaibani A, Hurley M, Potts M, et al. Diagnostic accuracy of high-resolution black-blood mri in the evaluation of intracranial large-vessel arterial occlusions. AJNR. American journal of neuroradiology. 2019;40:954-959
- Liu S, Tang R, Xie W, Chai S, Zhang Q, Luo Y, et al. Plaque characteristics and hemodynamics contribute to neurological impairment in patients with ischemic stroke and transient ischemic attack. European radiology. 2020
- Staessens S, Fitzgerald S, Andersson T, Clarençon F, Denorme F, Gounis M, et al. Histological stroke clot analysis after thrombectomy: Technical aspects and recommendations. International journal of stroke : official journal of the International Stroke Society. 2020;15:467-476
- Kang D, Jeong H, Kim D, Yang W, Lee S. Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibility-weighted magnetic resonance imaging. Stroke. 2017;48:1554-1559
Figures