0825
Evaluation of leptomeningeal collaterals by DSC-based signal variance and hemodynamic features in asymptomatic carotid artery stenosis1School of Medicine, Department of Neuroradiology, Technical University of Munich (TUM), Munich, Germany, 2MRRC, Yale University, New Haven, CT, United States, 3Philips Healthcare, Hamburg, Germany, 4Department of Neurology, Goethe University Frankfurt, Frankfurt, Germany
Synopsis
Detection of leptomeningeal collateral blood flow has high clinical relevance, but clinically applicable imaging methods are lacking. While a novel approach based on coefficient of variance (CoV) analysis of dynamic susceptibility contrast (DSC) MRI was recently proposed, relations to hemodynamic alterations remained unknown. Moreover, the role of leptomeningeal collaterals in internal carotid artery stenosis (ICAS) is under debate. We present multi-parametric hemodynamic evaluation within high CoV-voxels from 29 asymptomatic ICAS-patients and 30 age-matched healthy controls. Our results suggest no enhanced leptomeningeal collateral recruitment in asymptomatic ICAS. However, hemodynamic characteristics imply detection of voxels that are prone to future leptomeningeal recruitment.
Purpose
Leptomeningeal collateralization through pial vessels can compensate blood supply disruptions and is of major clinical relevance.1,2 On the one hand, it is known as an important protective pathway in acute stroke and has been associated with positive outcome prediction.2-4 On the other hand, leptomeningeal collateralization under sub-acute chronic hypoperfusion has been considered a negative indicator of severe hemodynamic compromise, but its role is still under debate.5-9 In internal carotid artery stenosis (ICAS), for example, some studies found associations with leptomeningeal collateralization,8-10 while others did not11.To overcome current methodological limitations to detect leptomeningeal collaterals, a novel approach was recently introduced based on common clinical dynamic susceptibility contrast (DSC) MRI.12 Here, voxels with elevated coefficient of variance (CoV) were proposed to reflect the degree of leptomeningeal collateralization. While first applications were promising,10,12 further validation is clearly demanded. And even though hemodynamic impairments in ICAS are well known,13,14 hemodynamic characteristics within voxels of elevated CoV remain uncertain.
The aim of our study was therefore to evaluate cerebral perfusion and oxygenation parameters within high-CoV voxels from DSC MRI in patients with asymptomatic ICAS in comparison with age-matched healthy controls (HC). We hypothesized novel insights to the CoV segmentation method itself and to collateral flow patterns in asymptomatic ICAS by additional multi-parametric hemodynamic evaluation.
Methods
Fifty-nine participants (29 asymptomatic, unilateral high-grade ICAS-patients, NASCET>70%, age=70.3±7.0y and 30 age-matched HCs, age=70.2±4.8y, see Tab.1) underwent MRI on a 3T Philips Ingenia (Philips Healthcare, Best, The Netherlands). The imaging protocol and derived parameters are summarized in Figure 1. Based on motion corrected DSC time-series data, spatial variance maps were calculated by $$CoV=\frac{\sigma}{\mu}$$ with the temporal standard deviation $$$\sigma$$$ and mean value $$$\mu$$$ for each voxel. High-CoV voxels were thresholded at the 70th percentile and ventricles as well as large vessels15 excluded by atlases to generate high-CoV masks according to Seiler et al.12 Multi-parametric hemodynamic imaging yielded maps of relative cerebral blood volume (rCBV) by DSC,16 cerebral blood flow (CBF) by pseudo-continuous arterial spin labeling (pCASL),17 and relative oxygen extraction fraction (rOEF) following the multi-parametric quantitative BOLD (mq-BOLD) approach.18 Processing was performed with SPM1219 and custom-built Matlab (Mathworks, Natick, USA) programs. Hemodynamic parameters were separately evaluated in both hemispheres of every participant within the high-CoV mask vs. all grey matter (GM) voxels. Two-sample t-tests were applied for statistical comparisons and considered significant for p<0.05.Results
Exemplary data is shown in Figure 2. Perfusion parameters were lateralized between hemispheres in ICAS (see Fig.2H) and symmetrical in HCs, as previously reported.13 In contrast, quantitative CoV values within high-CoV masks were symmetric between ipsi- and contralateral hemispheres in ICAS patients and comparable to HCs (Fig.3). Significant hemodynamic effects were found within high-CoV voxels compared to GM for all parameters (Fig.4). Specifically, CBF and rCBV were higher by approximately 14% (p<0.05; Fig.4A) and 20% (p<0.0001; Fig.4B), respectively, while rOEF was lower by -24% (p<0.0001; Fig.4C). These effects were highly consistent for all participants in both groups.Discussion
Our results indicate no enhanced recruitment of leptomeningeal collaterals in asymptomatic unilateral ICAS, as CoV values in both hemispheres were comparable to healthy controls. This is in agreement with previous studies in asymptomatic ICAS patients based on MRI9 and computed tomography11. In general, leptomeningeal collateral recruitment is only expected when primary collateral flow via the Circle of Willis fails.7,8 While perfusion impairments13 and shifted perfusion territories14 have previously been reported in our asymptomatic patient cohort, the hemodynamic compromise does not seem severe enough for leptomeningeal collateral recruitment.20 Interestingly, leptomeningeal collateralization has been reported in more severely affected symptomatic ICAS patients.9,10According to our results in both groups, high-CoV voxels are consistently characterized by high CBF and CBV, with concomitantly lower rOEF (see Fig.4), which indicates an elevated density of arterioles. This fits with the assumption that leptomeningeal collaterals primarily arise from arterioles1,2,21,22 and already exist before collateral recruitment23. Taken together, the applied CoV method might thus be sensitive to vessels at risk for future collateral recruitment in case of more severe of hemodynamic impairment.5,6
Furthermore, focally increased CBF in larger vessels has been attributed to arterial transit delay artefacts in another study via a similar CoV approach for ASL data.24 These transit delays have also been linked to collateral blood flow.25 Measured higher CBF in high-CoV voxels thus supports associations between high-CoV and collateral flow.
Quantitative evaluation of hemodynamic parameters within high-CoV voxels reveals higher differences in CBV (+20%) than CBF (+14%) compared to the surrounding tissue. This is in line with a previously reported decreasing ratio of CBF to CBV in high-CoV voxels, which was attributed to lower cerebral perfusion pressure (CPP).10 Following this argumentation, further CPP decreases may drive leptomeningeal collateral recruitment primarily in high-CoV voxels. This moreover supports the suspected detection of voxels at risk for future collateral recruitment by high CoV.
Conclusion
In the presented study, we successfully analyzed high-CoV voxels and multiple hemodynamic parameters in asymptomatic ICAS patients and healthy controls with two main implications. First, our results suggest no enhanced recruitment of leptomeningeal collaterals in asymptomatic ICAS. Second, hemodynamic characteristics point to identification of voxels with increased density of arterioles, that are prone to pial collateral flow in case of aggravation of hemodynamic impairment.Acknowledgements
We acknowledge support by Friedrich-Ebert-Stiftung (grant to SK), Dr.-Ing. Leonhard-Lorenz-Stiftung (grant SK 971/19) and the German Research Foundation (DFG, grant PR 1039/6-1).References
1. Brozici M, et al. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke 2004;34:2750–62.
2. Jung S, et al. Relevance of the cerebral collateral circulation in ischaemic stroke: time is brain, but collaterals set the pace. Swiss Med Wkly. 2017;147:w14538.
3. Menon BK, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterio circulation occlusions. AJNR 2011;32(9):1640-5.
4. Seners P, et al. Better Collaterals Are Independently Associated With Post-Thrombolysis Recanalization Before Thrombectomy. Stroke 2019;50(4):867-72.
5. Klijn CJ, Kappelle LJ. Haemodynamic stroke: clinical features, prognosis, and management. Lancet Neurol. 2010;9:1008–17.
6. Norrving B, et al. rCBF in patients with carotid occlusion. Resting and hypercapnic flow related to collateral pattern. Stroke 1982;13:155–62.
7. Powers WJ, et al. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med 1987;106(1):27-34.
8. Mueller M, Schimrigk K. Vasomotor Reactivity and Pattern of Collateral Blood Flow in Severe Occlusive Carotid Artery Disease. Stroke 1996;27(2):296-9.
9. Hartkamp NS, et al. Relationship between haemodynamic impairment and collateral blood flow in carotid artery disease. JCBFM 2018;38:2021-32.
10. Seiler A, et al. DSC perfusion-based collateral imaging and quantitative T2 mapping to assess regional recruitment of leptomeningeal collaterals and microstructural cortical tissue damage in unilateral steno-occlusive vasculopathy. JCBFM 2020; ePub ahead of print.
11. Dankbaar JW, et al. Internal Carotid Artery Stenosis and Collateral Recruitment in Stroke Patients. Clin Neuroradiol 2018;28(3):339-44.
12. Seiler A, et al. Signal variance-based collateral index in DSC perfusion: A novel method to assess leptomeningeal collateralization in acute ischaemic stroke. JCBFM 2020;40(3):574-87.
13. Kaczmarz S, et al. Hemodynamic impairments within individual watershed areas in asymptomatic carotid artery stenosis by multimodal MRI. JCBFM 2020; ePub ahead of print.
14. Kaczmarz S, et al. Increased variability of watershed areas in patients with high-grade carotid stenosis. Neurorad 2018;60(3):311-23.
15. Vinani R. A Digital Atlas of Middle to Large Brain Vessels and Their Relation to Cortical and Subcortical Structures. Front Neuroanat 2016.
16. Kluge A, et al. Analysis of three leakage-correction methods for DSC-based measurement of relative cerebral blood volume with respect to heterogeneity in human gliomas. MRM 2016;34(4):410-21.
17. Alsop D, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. MRM 2015;73(1):102–16.
18. Hirsch N, et al. Technical considerations on the validity of blood oxygenation level-dependent-based MR assessment of vascular deoxygenation. NMR Biomed 2014;27(7):853-62.
19. Statistical Parametric Mapping software (SPM12) Version 6225: http://www.fil.ion.ucl.ac.uk/spm. Wellcome Trust Centre, London, UK.
20. Liebeskind DS, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011;69:963-74.
21. Brozici M, et al. Anatomy and Functionality of Leptomeningeal Anastomoses A Review. Stroke 2003;34:2750-62.
22. Migrino RQ, et al. Human cerebral collateral arteriole function in subjects with normal cognition, mild cognitive impairment, and dementia. AM J Phys Heart Circ Phys 2018;315(2):H284-90.
23. Liebeskind DS. Collateral Circulation. Stroke 2003;34(9):2279-84.
24. Mutsaerts HJ, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. JCBFM 2017;37(9):3184-92.
25. Roach BA, et al. Interrogating the functional correlates of collateralization in patients with intracranial stenosis using multimodal hemodynamic imaging. AJNR 2016;37:1132-8.
26. Kaczmarz S, et al. Oxygen extraction fraction mapping with multi-parametric quantitative BOLD MRI: Reduced transverse relaxation bias using 3D-GraSE imaging. Neuroimage 2020;220:117095.Figures
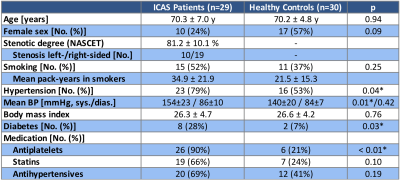

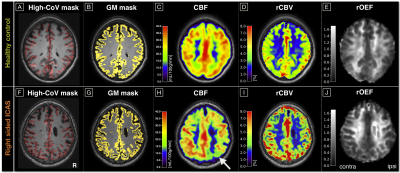
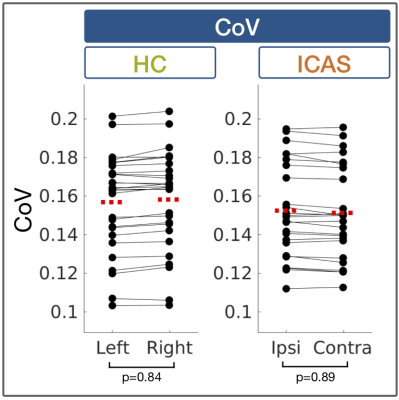
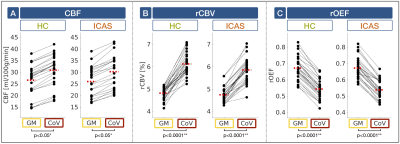
Figure 4: Hemodynamic characteristics in high-CoV voxels. CBF (A), rCBV (B) and rOEF (C) are compared in grey matter (GM; yellow) vs. high-CoV voxels (CoV; red) in healthy
controls (HC; green) and ICAS patients (orange). Dots show mean parameter
values, black lines connect the same subject’s mean values and red dashed lines
represent group average values. CBF was systematically lower due to background suppression13
and rOEF elevated due to T2 bias26. In high-CoV voxels of both groups, all parameters showed significant effects. While CBF (A) and rCBV (B) were higher, rOEF was
lower (C).