0824
Inner volume 3D TSE using optimized spatially selective excitation pulses for vessel wall imaging of intracranial perforating arteries at 7T1Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 2MR Collaboration, Siemens Healthcare Ltd, Beijing, China, 3Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China, 4The Innovation Center of Excellence on Brain Science, Chinese Academy of Sciences, Beijing, China
Synopsis
The impairment of microvessels can lead to neurologic diseases such as stroke and vascular dementia. The imaging of lumen and vessel wall of perforating arteries requires an extremely high resolution due to their small caliber size. In this study, we developed a 3D inner-volume (IV) TSE (SPACE) sequence with optimized spatially selective excitation (SSE) RF pulses. High resolution of isotropic 0.30mm within ten minutes was achieved for the black- blood images of lenticulostriate artery (LSA) for the first time. The IV-SPACE images showed clearer delineation of vessel wall and lumen of LSA than conventional SPACE images.
Introduction
The evaluation of perforating arteries is important for the diagnosis of small vessel disease1. However, there is no suitable MRI imaging technique for both the lumen and vessel wall of perforating arteries because of their small caliber size (50 – 400 um), which requires a high spatial resolution. In this study, we developed a 3D inner-volume (IV) TSE (SPACE) sequence with optimized 2D spatially selective excitation (SSE) RF pulses. The optimized SSE RF pulses were designed through a series of optimization including iterative RF pulse design, trajectory optimization, and phase convention of Carr-Purcell-Meiboom-Gill (CPMG) condition to meet the perforating arteries imaging demands. High resolution of isotropic 0.30mm within ten minutes was achieved for the vessel wall imaging of lenticulostriate artery (LSA) for the first time. The imaging results from the optimized RF pulse was compared to the traditional 2D RF pulse. The LSA vessel wall imaging from IV-SPACE sequence was also compared with conventional SPACE.Methods
The designed SSE RF pulse was incorporated into a 3D TSE sequence (named SPACE by Siemens) to produce the IV-SPACE sequence. The traditional 2D SSE RF pulse2 was designed with a constant angular rate spiral-in k-space trajectory. The optimized SSE RF pulses were calculated following the method described in Yip et al3, and CPMG condition and variable-density spiral-in trajectory were additionally enrolled to optimize SSE RF pulse. The variable-density spiral-in trajectory was designed to adequately sampled excitation k-space near the origin (XFOV=25cm), and under-sampled in the high-frequency region (XFOV=6cm). Weighting factor Wr=0.25 and regularization parameter λ=100 was used for RF pulse designs. B0 and B1 maps were collected to design an optimized pulse. The scan of 10 volunteers was performed on a human 7T MR research system (Siemens Healthcare, Erlangen, Germany) with a Nova 32-channel head coil. The number of stems and branches of LSA were counted. Wilcoxon signed-rank test was used to compare the numbers of stems and branches between the pairwise comparison of the three imaging methods. The intraclass correlation coefficient (ICC) was calculated and reported for the measurements of LSA.Results
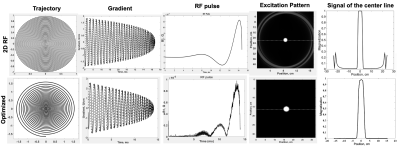
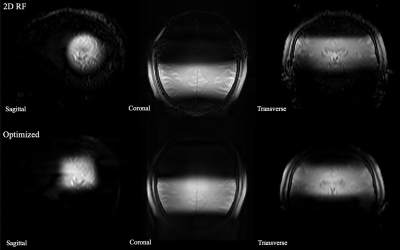
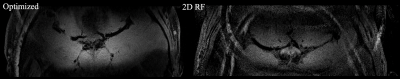
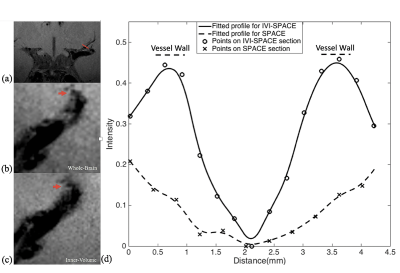
The designed RF pulse and Bloch simulation of the traditional 2D RF pulse and optimized RF pulse were shown in figure 1. The optimized pulse was shorter than the traditional pulse (14.10ms vs. 16.75ms). Figure 2 showed the typical excitation pattern of the SSE pulses in a low-resolution scan across the entire brain, which revealed that the optimized RF pulse had better suppression of signals outside the ROI. The 0.3mm isotropic images obtained by the optimized RF pulse and the traditional 2D RF pulse were shown in Figure 3. Coronal MinIP (projection thickness = 18 mm) images were shown in figure 4 for 2D RF (a), optimized method (b), and conventional SPACE (c). There was no significant difference in the numbers of stems visualized among three different methods. The number of branches visualized by 2D RF and conventional SPACE were comparable, and both them were significantly lower than that by optimized method (Optimized vs. Conventional SPACE = 7.8±1.54 vs. 7.1±1.33, p = 0.002, ICC = 0.817; 2D RF vs. Optimized = 7.0±1.78 vs. 7.8±1.54, p = 0.001, ICC =0.805;). The LSA vessel wall was obvious in IV-SPACE (red arrow) but almost invisible in the conventional SPACE image in Figure 5. With a cut line through the vessel center, IV-SPACE images showed a significant signal drop at the lumen, which was almost absent in conventional SPACE images.Discussion
This work successfully produced a clear vessel wall image of the proximal LSA. This is the first time intracranial black-blood images with isotropic 0.30 mm resolution within ten minutes have been demonstrated. The optimized SSE RF pulse is robust, and no obvious signal folding from outer excitation FOV is observed. The results demonstrate that IV-SPACE imaging of the LSA vessel wall is feasible in a population of healthy volunteers. The numbers of stems of LSAs visualized by three methods showed no significant difference, demonstrating comparable abilities of these methods on displaying primary structures of LSAs. The higher number of branches of optimized methods may be due to the higher resolution reveals much more details. The lower number of branches in 2D RF methods may be due to interference of aliased signals caused by incomplete signal suppression outside ROI (Figure 4, white arrows). A higher spatial resolution reduces the partial volume effect, so the LSA delineation is significantly improved in the IV-SPACE images (Figure 4, yellow arrows). The reduction of the echo train length from 50 to 30 might also contribute to the sharpness of the vessel walls due to less signal decay (Figures 5). The sharper vessel wall potentially benefits the diagnosis and also the study of cerebral vascular diseases. The clinical implication of this technique on small vessel disease will be explored in the future.Conclusion
The inner-volume 3D TSE sequence was developed to achieve isotropic 0.30 mm black-blood images within a clinically acceptable time. The optimized SSE RF pulse is robust, and achieve better background signal suppression. A higher resolution produces sharper delineation of the vessel wall and lumen of intracranial perforating arteries. The technique is promising for the evaluation of microvasculopathies of cerebral vascular diseases.Acknowledgements
This study has received funding from the National Natural Science Foundation of China (82001804, 8191101305), Ministry of Science and Technology of China (2017YFC1307904), Natural Science Foundation of Beijing Municipality (7191003), Strategic Priority Research Program of Chinese Academy of Science (XDB32010300).References
1. Greenberg, S.M., Small vessels, big problems. N Engl J Med, 2006. 354(14): p. 1451-3.2.
2. Pauly J, Nishimura D, Macovski A. A k-space analysis of small-tip- angle excitation. J Magn Reson 1989; 81:43–56.
3. Yip C, Fessler J A, Noll D C. Iterative RF pulse design for multidimensional, small-tip-angle selective excitation[J]. Magnetic Resonance in Medicine, 2005, 54(4): 908-917.
Figures